By far the most important cyanides used in cyanidation are sodium cyanide and calcium cyanide. The latter product is sold in an impure form analyzing close to 50% NaCN equivalent. The former is sold in various grades from 85 to 98% NaCN.
In comparing the dissolving effects of the cyanides of ammonium, sodium, potassium, magnesium, calcium, strontium and barium on gold and silver it was found that the alkaline radical did not affect the dissolving effect of the particular cyanide. The cyanogen content was the only significant factor affecting dissolution. For example, 1 mol of pure calcium cyanide weighing 92 grm. would dissolve the same amount of gold or silver as 2 mols of pure sodium cyanide weighing 98 grm. because their CN content is the same in each case, i.e. 52 grm.
Decomposition of Cyanide Solutions
A water solution of an alkaline cyanide hydrolyzes as follows:
NaCN + H20 <–> HCN + NaOH
The extent to which this hydrolysis proceeds in solutions of commercial cyanides in water depends primarily on the amount of free alkali in the cyanide. If this alkali is appreciable, then the decomposition of cyanide might be negligible. In the absence of appreciable free alkali, hydrolysis can be retarded by the addition of lime. In practice the addition of lime to a cyanide pulp is practically universal, not only to prevent loss of cyanide by hydrolysis but also to neutralize any acidic constituents of the ore which otherwise would liberate hydrocyanic acid. Another factor affecting decomposition of cyanide solutions is the presence of carbon dioxide in the air. The carbonic acid, being stronger than hydrocyanic acid, decomposes alkaline cyanide solutions as follows:
NaCN + H2COS = HCN + NaHCO3
This reaction, also, can be prevented by the use of lime or other alkalies. Such alkalies maintain the alkalinity of the solution and react with the carbon dioxide to form innocuous compounds such as calcium carbonate.
|
EFFECT OF LIME ON CYANIDE LOSS |
||||
| No Lime |
Lime |
|||
|
Start |
Lime After 6 Hour | Start |
Lime After 6 Hour |
|
|
NaCN% |
0.051 |
0.034 | 0.051 |
0.051 |
|
CaO% |
0.001 |
0.0005 | 0.061 |
0.015 |
|
pH |
10.2 | 9.7 | 11.6 |
11.2 |
| NaCN Loss lb/ton | 0.34 |
Nil |
||
The result of tests shown in above demonstrate the effect of alkalinity on loss of cyanide by hydrolysis and by the action of carbon dioxide in the air. Two solutions were prepared, each containing 0.051% NaCN; 0.061% CaO as hydrated lime was added to one, while the other was left in its natural, slightly alkaline state. The solutions, at a temperature of 24 C. were agitated in wide mouthed open bottles on rolls for 6 hours after which they were titrated for free cyanide and lime content. The pH of each solution was determined before and after the test. From the results it may be noted that during the test the solution without lime varied in pH from 10.2 to 9.7; in 6 hours this solution lost 33% of its cyanide, or 0.34 lb. NaCN per ton. In the case of the other solution where the pH varied from 11.6 to 11.2 the loss of cyanide was nil.
Dissolution of Precious Metals
The following reactions have been given for the dissolution of gold in dilute cyanide solutions: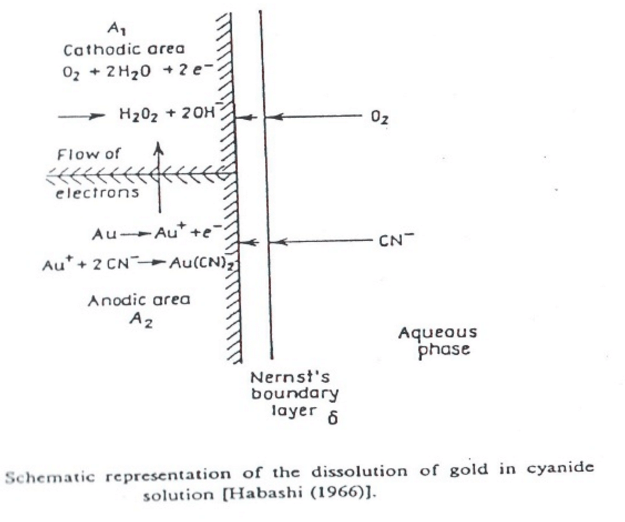
4Au + 8 NaCN + 02 + 2 H20 = 4 NaAu(CN) 2 + 4NaOH.
This is known as Eisner’s equation.
2Au + 4 NaCN + 2 H20 = 2NaAu(CN)2 + 2 NaOH + H2.
This was suggested by Janin.
2Au + 4 NaCN + 2 H20 + 02 = 2 NaAu (CN)2 -f- 2 NaOH -f- H202.
The hydrogen peroxide formed being used in the reaction:
2 Au + 4 NaCN + H202 = 2 NaAu(CN)2 + 2 NaOH.
These reactions were suggested by Bodlaender. The overall equation, however, is the same as Eisner’s.
Analogous equations have been given for the dissolution of metallic silver in cyanide solutions.
Barsky, Swainson and Hedley determined the free energies of formation of the complex gold-cyanide and silver-cyanide ions. From the data obtained, they calculated the free-energy changes in the various reactions suggested and pointed out which of these are theoretically pos-sible under ordinary cyanidation conditions.
Their results showed that for Eisner’s equation the reaction will proceed practically to completion, i.e. until practically all the cyanide has been consumed, or all of the metal dissolved.
For Janin’s equation the equilibrium constants are so very unfavorable that the formation of hydrogen may be considered impossible under ordinary cyanidation conditions.
For Bodlaender’s equations the equilibrium constants are favorable, therefore, the reactions proposed are possible. In this connection Bodlaender actually found that hydrogen peroxide was formed and he was able to account for approximately 70 per cent of the theoretical amount of hydrogen peroxide that should be formed according to his equation. In view of this, it would appear that Bodlaender’s equations express the true reactions that take place when metallic gold and silver are dissolved in dilute cyanide solutions.
Effect of Cyanide Concentration on Rate of Gold and Silver Dissolution
The rate of dissolution of gold in cyanide solutions attains a maximum in passing from concentrated to dilute solutions. His work shows that this maximum is reached at a solution concentration of 0.25% NaCN.
According to Christy, for all practical purposes, solutions weaker than 0.001 per cent KCN do not dissolve gold.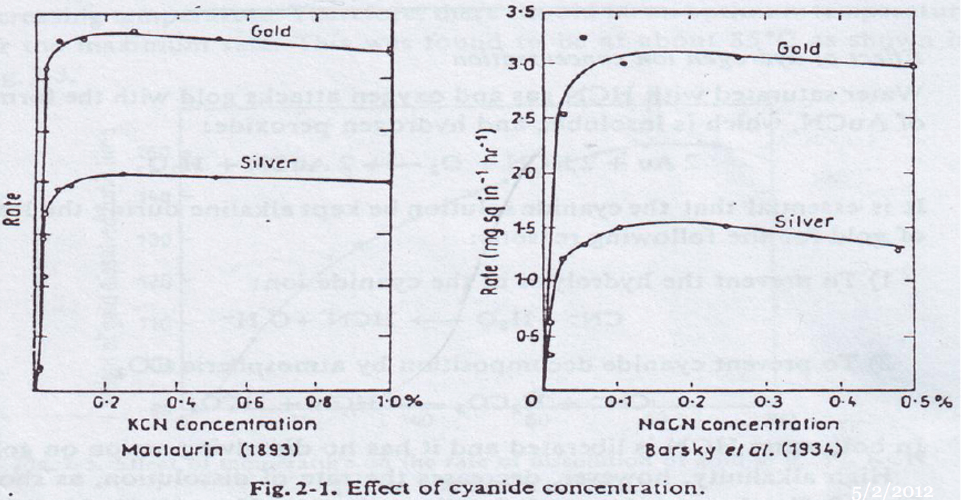
Research found that the rate of dissolution of gold increased rapidly with increase in strength of solution up to and including 0.10 per cent KCN.
White found that maximum rate is at about 0.027 per cent KCN, or 0.020 per cent NaCN, when the cyanide solution is saturated with oxygen.
The solution strength found for most rapid dissolution was 0.05 per cent NaCN. .
The cause of the wide variations in the solution strengths found by various investigators to give maximum rate of gold dissolution probably lies in the variety of techniques employed in determining these figures. These variations include such factors as the ratio of volume of solution to gold surface. violence of agitation, rate of aeration. If a large volume of cyanide solution is used and a relatively small surface of gold exposed to the cyanide solution and if agitation is sufficiently intense to remove the products of the reaction from the surface of the gold as rapidly as they are formed, then the controlling factor governing the rate of dissolution of the gold should he the oxygen concentration of the solution in contact with the gold. If air is used and the tests are run at sea-level the maximum concentration of oxygen in solution will he 8 mg per liter. Then according to the reaction:
4 An + 8 NaCN + 0, + 2 H,0 =
392 32
4NaAu(CN), + 4 NaOH,
there would be no advantage in having more than 392 parts by weight of NaCN for every 32 parts by weight of oxygen or 98 parts of NaCN for every 8 parts of oxygen in the cyanide solution. In other words, the maximum rate of dissolution of gold under ideal conditions of agitation and aeration should take place in solutions containing 0.0098 per cent NaCN. In support of this, Hedley and Kentro found that by using 10 sq. cm. of gold in 1 liter of cyanide solution and aerating vigorously (28 liters per hour), the maximum rate of dissolution took place between 0.011 and 0.051 per cent NaCN; the rate of dissolution in the former solution was 95′ < of the maximum.
In practice most cyanide plants treating gold ores use solutions containing less than 0.05 per cent NaCN: the general average is probably in the neighborhood of 0.02 to 0.03 per cent NaCN.
The maximum rate of dissolution of metallic silver in cyanide solutions took place at about 0.10 per cent NaCN. This, of course, does not apply to the dissolution of silver sulphide minerals, because a different set of conditions is involved.
Relative Rates of Dissolution of Gold, Silver and Their Alloy
Using 100 ml. volumes of 0.10 per cent NaCN solution, with surface areas of the metals and their alloys measuring 10 sq. cm., and with a constant volume of air for aeration and agitation, it was determined the relative rates of dissolution of gold, silver and two gold-silver alloys. From these results it may be observed that silver dissolved at about half the rate at which gold dissolved; the rates of dissolution of the alloys were between those of gold and silver, almost in proportion to the composition of the alloys. The amounts of gold and silver dissolved out of the alloys were in practically the same proportion as the percentages of those metals in the alloys.
|
Relative Rates Of Dissolution Of Gold, Silver And Gold-Silver Alloys |
||||
|
Analysis of Original Metal |
Dissolution Rate mg/sq. cm/hr |
Analysis of Dissolved Metal |
||
|
Au % |
Ag% |
Au% |
Ag% |
|
|
100 |
— | 2.99 | 100 | — |
|
79.8 |
20.2 | 2.44 |
78.6 |
21.4 |
|
57.6 |
42.4 | 1.94 | 56.5 |
43.5 |
|
— |
100 |
1.54 |
— |
100 |
https://www.academia.edu/292086/Kinetics_and_Mechanism_of_Gold_and_Silver_Dissolution_In_Cyanide_Solution
https://open.library.ubc.ca/cIRcle/collections/ubctheses/831/items/1.0106728
