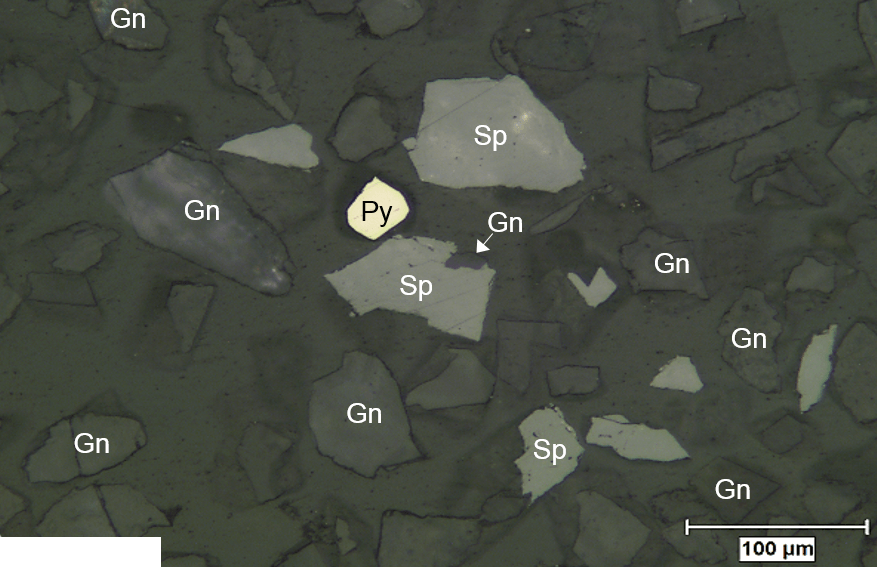
A Mississippi Valley Type Deposit or commonly called VMT offers some of the simplest, cleanest and “easiest” mineralogy and therefore metallurgy in the world.
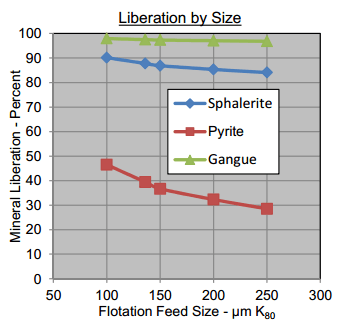
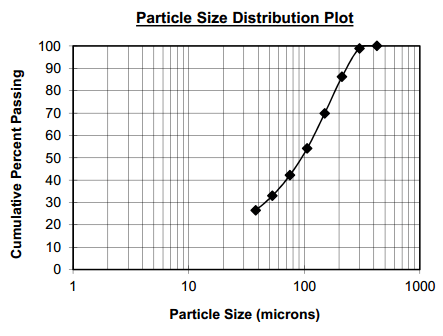
As shown in the image below, a near perfect liberation of all minerals at 100 microns grind.
As seen below, Mississippi Valley Type Deposits liberate very well as coarse grinds. In the example P80 of almost 200um is satisfactory.
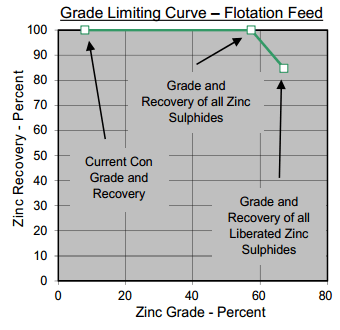
The liberation of the resulting zinc concentrate are often excellent and have been seen to measure up to 96 percent. On samples of both flotation feed and the final concentrate, the majority of the unliberated sphalerite was interlocked with non-sulphide gangue. These binary particles in the feed contained about 47 percent sphalerite and should be recoverable in rougher flotation.
The main diluent in the final concentrate was non-sulphide gangue. Almost half of these non-sulphide gangue particles were liberated particles measured in the finest size fraction less than 9pm; this was likely the result of entrainment.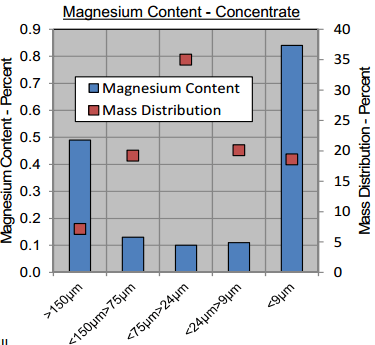
Often, MVT Mississippi Valley Type Deposits (for being hosted in Dolomite, will be rich in MgO. In this case, Magnesium content in each of the tested fractions of the concentrate sample did not measure higher than 1 percent. The highest magnesium content was measured in the coarsest and finest fractions measuring about 0.5 percent and 0.8 percent, respectively with an overall average of 0.3% Mg.
Since Zinc Sphalerite (Zn,Fe)S is 64.06 % Zinc and Mississippi Valley metallurgy often gives a zinc conc grade of 63%, nobody worries much about mineral processing hardship in an MVT flotation plant.
The majority of the diluents in the these concentrates are physically locked with sphalerite. In this zinc concentrates, within the size ranges examined during the modal study, very few particles of liberated pyrite or non-sulphide gangue were detected.
Based on an analysis of the available modal data, regrinding down to about 50 um P80 would be required to significantly change the liberation levels of the diluents in the zinc concentrates.
There exists no evidence to demonstrate that dilution cleaning alone would significantly change the pyrite and the non-sulphide gangue levels in the current zinc concentrates.
In these modal studies, only the coarser particle size ranges of each sample were scanned. Approximately 45 percent by weight of each zinc concentrate samples was not studied by these modal methods. It is possible that evidence of the presence of entrainment mechanisms may be revealed, on very fine particle size ranges.