As sphalerite is such a common constituent of many types of ore, the present investigation was undertaken to determine what its relations are to the other minerals in the deposits, and also whether these are of any genetic significance.
The data presented herewith were obtained from a mineralographic study of about 200 ore specimens from some 43 different localities, represented by suites of specimens in the Cornell University collection. These, grouped according to their genesis, were as follows:
I. Contact-metamorphic Deposits.-—Silver City, N. M.; Kelly, N. M.; Silver Bell, Ariz.; Bingham, Utah; Agaguca, Michoacan, Mex.; Mate- huala, Mex.; Sombreretti mine, Mex.
II. Deep-vein Zone Deposits.—-Mineral, Louisa Co., Va.; Edwards, N, Y.; Chestnut Yard, Va.; Allah Cooper mine, Louisa Co., Va.; Globe, Ariz.; Moyie, B.C.; Rossland, B.C.;Broken Hill, N.S.W.; Weedon, Que.
III. Intermediate-vein Zone.—Park City, Utah; Leadville, Colo.; Butte, Mont.; Santa Barbara, Mex.; Red Cliff, Colo.; Burro Mountains, N. M.; Idaho Springs, Colo.; Blue River, Ore.; Rye, Colo.; Slocan district, B, C.; Freiberg, Ger.; Clausthal, Ger.
IV. Shallow-vein Zone.—Ouray, Colo.; Goldfield, Nev., Zacatecas, Mex.; Field, B. C.; Kapnik, Austria; Frontenac mine, Kingston, Ont.
V. Meteoric Water Deposits.—Joplin, Mo.; Southwest, Wis.; Austinville, Va.; Crittenden County, Ky.; Ridgeburg, Pa.; Moresnet, Belgium.
It is not supposed that the investigation of such a limited number of occurrences may warrant conclusive and universal generalizations, but the conclusions reached are believed to be reliable in so far as the specimens that were examined are concerned.
SUMMARY OF RESULTS:
The main facts brought out by the study of the blende-bearing ores examined are as follows:
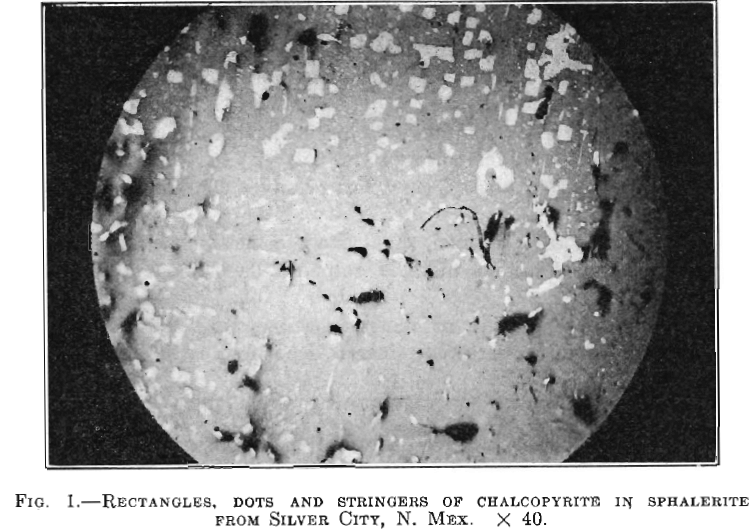
1. Chalcopyrite as minute triangular or rectangular dots, or as stringers, can be practically always found in sphalerite that has been deposited by ascending juvenile waters, the amount apparently varying somewhat directly with the temperature and pressure of formation. The dots show a strong tendency to group themselves along crystallographic directions.
2. Chalcopyrite, as minute dots or stringers was not found in sphalerite deposited by meteoric waters.
3. Sphalerite does not appear to carry silver compounds in visible amounts.
4. In all the ores examined, sphalerite is generally the first valuable sulphide and the second metallic sulphide deposited by ascending solutions.
5. Sphalerite deposited by meteoric waters does not occupy any definite position in the series of minerals deposited.
General Occurrence Of Sphalerite:
Sphalerite has been observed to occur in nature, as an original constituent of granite ; in vein deposits of all zones, in contact-metamorphic deposits, and in those formed by the action of meteoric waters. When deposited by solutions or vapors its precipitation may take place in cavities or by replacement.
One odd occurrence is in lignite, and another as nodules in clay under a Michigan coal.
Even in deposits of the same type, the occurrence of sphalerite may be somewhat variable.
In taking up the present discussion, it seems best to consider the deposits in groups of similar genetic character.
Contact-Metamorphic Deposits:
In the specimens examined, the sphalerite carries chalcopyrite, usually in the form of minute triangular, rectangular, or rounded dots, as well as stringers, which often follow crystallographic directions, their presence being most pronounced. These can usually be seen with a low-power objective (Fig. 1), but the use of a higher power often discloses great quantities of smaller dot clusters (Fig. 2). In many cases, larger areas of chalcopyrite may be present in the sphalerite, in addition to the dots, but this is the exception rather than the rule. Galena and pyrrhotite rarely occur in the same relation as the chalcopyrite, but
a case of the former was observed in some ore from Bingham Canyon, Utah, and of the latter from the Matehuala district, Mexico.
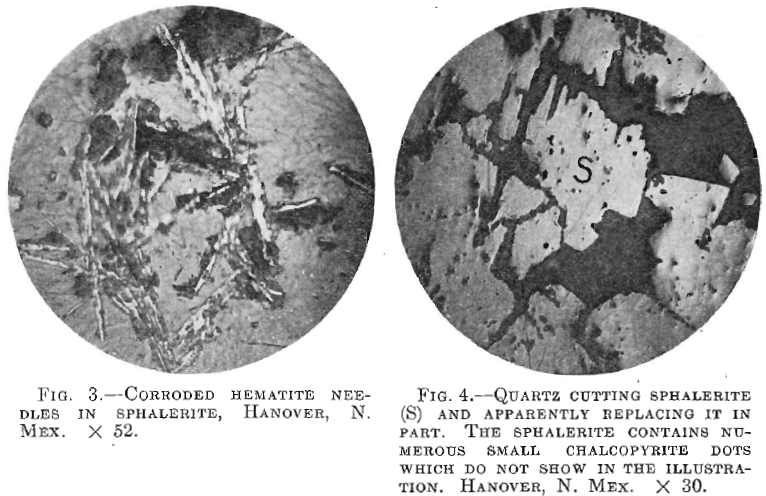
Pyrite, when present, seemed invariably to be the first sulphide deposited, while sphalerite in every case, except two, followed it. Thus at Matehuala, the pyrrhotite may have been earlier than the sphalerite, while at Hanover, N. M., the occurrence of corroded hematite needles in sphalerite seems to indicate a replacement of the former by the latter (Fig. 3).
The primary associates of the sphalerite in the specimens examined were restricted to chalcopyrite, galena and pyrrhotite, named in the
order of their importance. Pyrite at times seems undoubtedly replaced by sphalerite, but it also replaced gangue minerals.
In one specimen from Hanover, N. M. (Fig. 4), the sphalerite appeared to be replaced by quartz. The reasons suggesting this are that the quartz not only cuts the sphalerite in veins, but also penetrates it in a manner suggesting replacement.
Deep-Vein Zone Deposits:
In this group, as compared with the preceding, there seemed to be a marked decrease in the intensity and regularity of distribution of the chalcopyrite dots within the sphalerite, and in some the dots appeared to be absent, but this may have been a local exception, or the grains of the specimen examined only may have been free from it.
The presence of pyrrhotite seemed to be unfavorable to the development of chalcopyrite in sphalerite since it was often found in areas in which the former was lacking. This absence of chalcopyrite dots in the sphalerite might be explained, however, by assuming an order of crystallization different from that usually observed. Thus in specimens from the LeRoy mine at Rossland, B. C., the chalcopyrite appeared to replace pyrrhotite, while the small amount of sphalerite present was evidently later, but contained some small patches of pyrrhotite. It is possible that these are unreplaced fragments of the latter in the former. At this locality it seems that both pyrite and pyrrhotite at least, may be of several different generations, a fact that has been noted by both Drys-
dale and Bruce. Each of these writers also notes that the sphalerite postdates the chalcopyrite.
When pyrite was present it was almost universally the first sulphide deposited. It was then followed by sphalerite, in one instance closely attended by pyrrhotite.
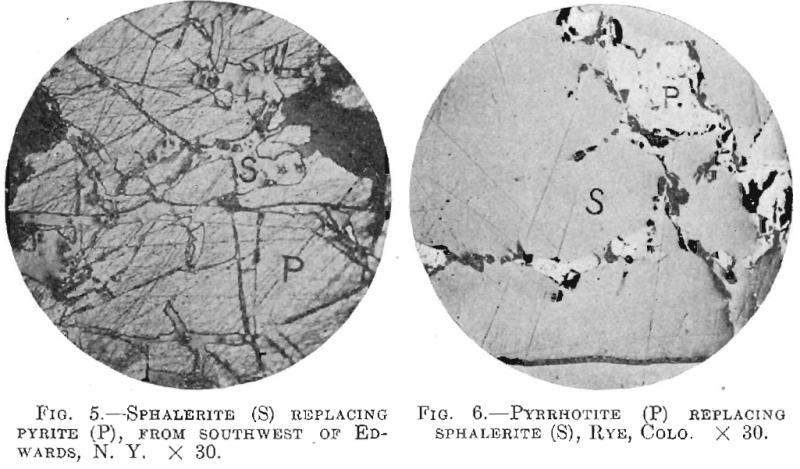
The sphalerite is replaced by chalcopyrite and galena, and was found replacing both quartz and pyrite, a good occurrence of the last being shown by ore from southwest of Edwards, N. Y. (Fig. 5).
Intermediate-Vein Zone:
The development of chalcopyrite dots and stringers in the sphalerite was noticed in all specimens examined, but in those from the Ontario mine, Park City, Utah, they were by no means abundant. On the whole, the chalcopyrite dots seemed to be slightly more numerous than in ores of the deep zone, although this may have been merely a coincidence.
Wherever pyrite was present, it was found to antedate the sphalerite, the period of deposition of the two apparently being sometimes separated by that of gangue minerals.
Following the sphalerite came chalcopyrite, sometimes accompanied by pyrrhotite and after this galena followed by tetrahedrite.
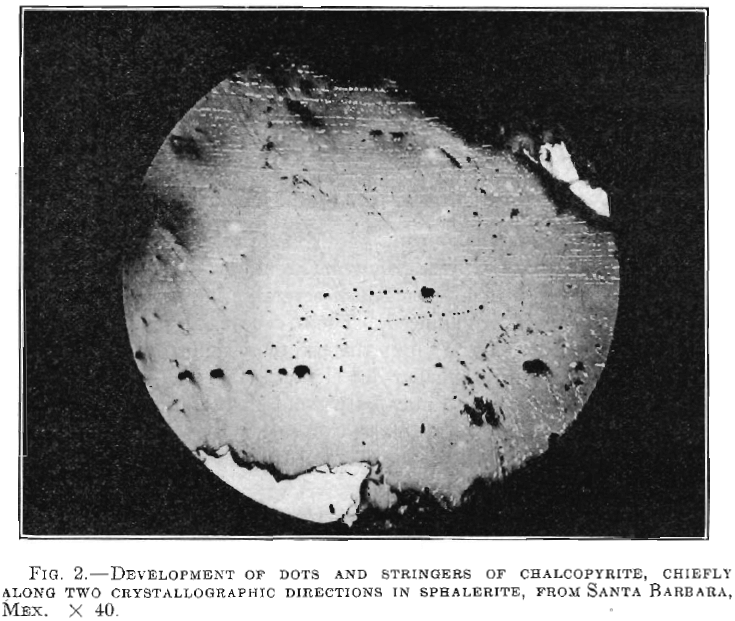
Galena, tetrahedrite, chalcopyrite and possibly pyrrhotite were seen replacing the sphalerite, while the last named was found replacing pyrite, schist and some of the gangue minerals. Fig. 2 shows a remarkable and uniform development of chalcopyrite, from Santa Barbara, Mex. Fig. 6, from a vein in gneiss near Rye, Colo., shows sphalerite cut and replaced by pyrrhotite.
Shallow-Vein Zone:
Although the chalcopyrite dots were by no means lacking in ores of this group, there seemed to be a decided decrease in their number and size; neither was their persistence along cleavage directions in the sphalerite as striking as in the preceding groups.
Pyrite, when present, was the first sulphide to form, but when pyrite was absent sphalerite was the first. Chalcopyrite appears in some cases to be contemporaneous with the galena, and may occur in the same veinlets with it. Tetrahedrite is common, but was a late precipitation. It is more closely associated with the galena than with the sphalerite.
In general, the galena and tetrahedrite replace the sphalerite.
In one specimen from Kapnik, Hungary, the chalcopyrite in medium¬sized areas is in contact with the sphalerite, while in the latter, near the boundary, are many minute dots of the former. These dots also occur in the sphalerite, on either side of chalcopyrite veinlets.
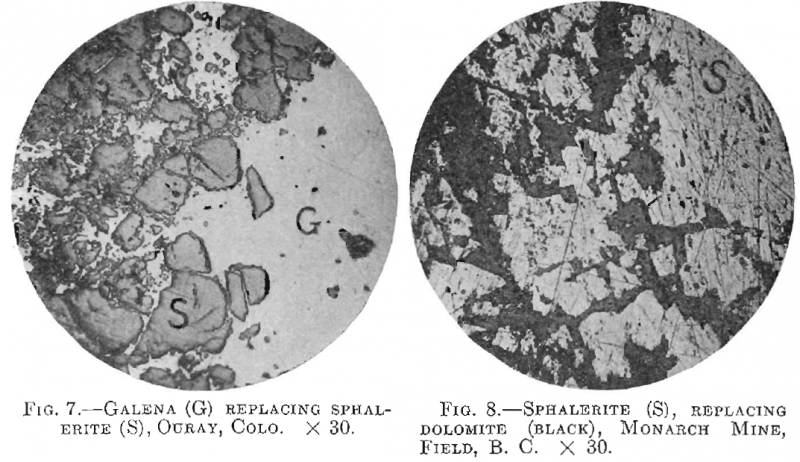
A good example of replacement of sphalerite by galena is shown in Fig. 7 from Ouray, Colo.
Fig. 8 is a specimen from the Monarch mine at Field, B. C., showing the replacement of dolomite by sphalerite. No intrusive rock is found near the orebody, but the sphalerite contains dots of chalcopyrite.
Deposits from Meteoric Waters:
The chalcopyrite dots and stringers found almost constantly in ores deposited by ascending solutions seem to be entirely lacking in those ores whose concentration is commonly regarded as the work of meteoric waters.
In most of these deposits, chalcopyrite is entirely absent, but in some specimens from Joplin, Mo., sphenoids of chalcopyrite were noticed both on the outside of the sphalerite crystals and within them.
The order of crystallization in this group of deposits appears to be indefinite. Sphalerite is often the first valuable sulphide to crystallize, and at times may precede the marcasite. In other instances, galena seemed to have preceded the sphalerite, but at other times their relationship was not capable of definite interpretation. Again, at others there may be an alternation of the zinc and iron sulphides as at Moresnet, Belgium.
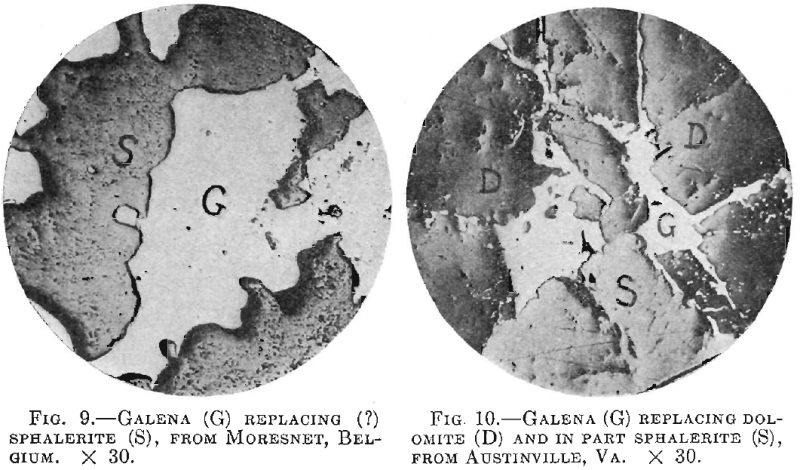
Galena occasionally appears to replace the sphalerite, and this sometimes seems to be true of specimens that show a crustified structure. Thus Fig. 9 is from a specimen of crustified ore from Moresnet, Belgium.
The specimen is made up of alternating bands of zinc sulphide and iron sulphide, but along certain bands of the former the hand specimen shows grains of galena. Under the microscope, however, the relation of the two is such as to show that the galena may in part replace the sphalerite.
In Fig. 10, both sphalerite and galena replace the dolomite, but the second is later than the first.
Fig. 11 shows sphalerite from Joplin, Mo., containing triangular crystals of chalcopyrite, but no dots of the latter, and the crystals are around the edge of the sphalerite grains.
Discussion of Results:
In the foregoing statements, a few facts of importance seem to stand out and warrant further comment, viz:
- The occurrence of chalcopyrite as dots or stringers in the sphalerite.
- The comparative absence of many minerals as primary associates of the sphalerite.
- The prevalence of sphalerite as the first valuable sulphide to crystallize or as the second metallic sulphide.
- The replacement of sphalerite by metallic minerals.
- The possible replacement of sphalerite by non-metallic minerals.
These may be considered in more detail:
1. Chalcopyrite Impregnations—-The chalcopyrite dots so widely noticed in this paper have been casually mentioned by several writers. Thus Dolmage found them in the ore from Tyee, Vancouver Island; Graton and Murdock also observed them from several localities. They were likewise noted by Wolcott, in ores from Clear Creek County, Colo.; by Thompson, from Ducktown, Tenn.; by Overbeck in Maryland ores; and by Guild in blende-bearing silver ores.
If, then, there is such a widespread occurrence of copper in sphalerite, one would expect to find it in analyses of the latter mineral, and such is the case.
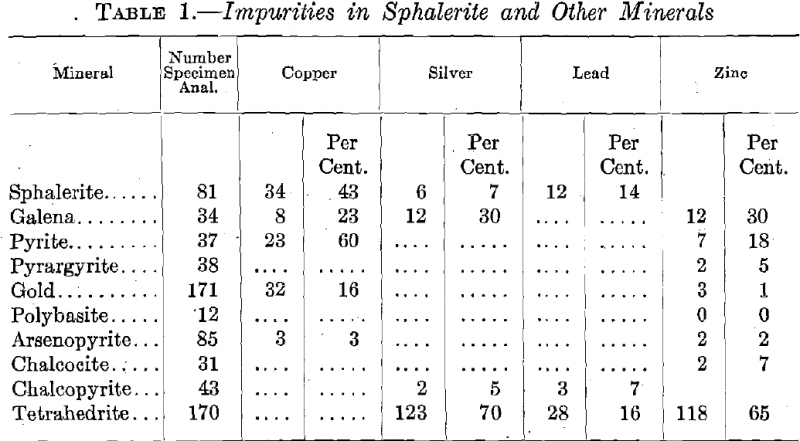
In Table 1, compiled from analyses quoted by Hintze, there are given a list of a number of common metallic minerals, the number of analyses, and the percentage of these showing copper and other impurities which bring out the fact that it is more abundant in pyrite and sphalerite than any of the others.
It is not known, of course, whether these impurities represent replacements, mechanical inclusions or intergrowths.
If we could eliminate all those sphalerite analyses representing occurrences deposited by meteoric waters, the percentage of cupriferous sphalerite would undoubtedly be much greater. We do not know, of course, the exact relation of the copper to the mineral analyzed in each case, but its affinity for zinc seems rather striking, and is even more pronounced in the microscopic study of the ores.
Considering the relation existing between the relative development of chalcopyrite and the genetic type of ore, we see from Table 2 that the contact-metamorphic deposits showed an excellent development of the dots with a decreased occurrence in the deep-vein zone type, and an increase again in the intermediate-vein zone type, while in the upper- vein zone ores, the dots were much less numerous, and in some cases required a high power for their recognition.
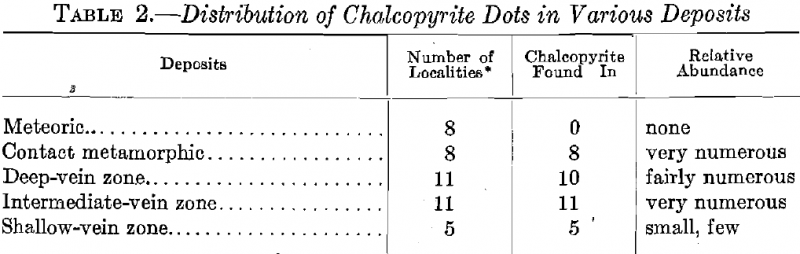
At least two polished specimens were examined from each locality.
In the deposits concentrated by meteoric waters, the absence of the dots is remarkable, even though some of the deposits contained chalcopyrite.
In this connection, it may be of interest to refer to the case of the Frontenac mine, near Kingston, Ont.; regarding whose origin some doubt has been expressed. Uglow suggests that the fluorite found in the group of veins of which this is one may be of magmatic origin, while the calcite, galena, and sphalerite have been obtained from the surrounding rocks. The sphalerite, however, shows dots of chalcopyrite, but not in abundance.
We may well ask why the chalcopyrite seems to favor the sphalerite, often to the exclusion of other equally available minerals. There seems to be nothing in the affinity of copper, or of copper and iron, for zinc, to warrant it, and nothing experimental seems to have been done along this line.
Galena is certainly as brittle as sphalerite, and offers more ready access for solutions along its cleavages, yet rarely does chalcopyrite penetrate galena to any extent in the dot form, though it may sometimes be later than the galena. That chalcopyrite is not entirely averse to lead is shown by the fact that it may be sometimes associated with galena, and galena with chalcopyrite enter together in many upper-zone deposits.
In order to find out whether porosity might be a factor in causing the association of sphalerite and chalcopyrite, a determination of the porosity of selected specimens of galena and sphalerite from Joplin, Mo., was made, for it was thought that since the galena is the most common associate of the sphalerite, it would be the best mineral with which to make a porosity comparison.
The results are as follows:
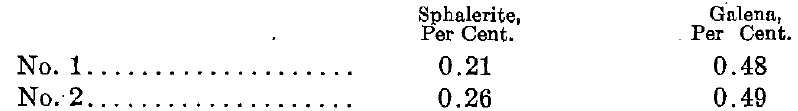
From this we see that as far as the relative porosities are concerned the galena affords the better host for the chalcopyrite. Hence we must conclude that the porosity of the sphalerite has nothing to do with its impregnation by chalcopyrite.
In the case of ores deposited by ascending waters, it is probable that the presence of heat and pressure may aid the chalcopyrite to penetrate the sphalerite, and replace it. This it does along sub-microscopic channels, being deposited either in straight lines (Fig. 2), or in spots (Fig.1), Sometimes the spots taper off along the lines on which they entered. Since the chalcopyrite dots are more abundant in the deep-vein zone deposits, it would seem that heat and pressure are a factor governing the extent of the impregnation.
In the case of deposits concentrated by meteoric waters, we are dealing with solutions under less temperature and pressure. Such solutions might not, therefore, have the power to easily impregnate the dense sphalerite, even though they carried copper.
As a matter of fact, the copper content of this type of deposits is small, although excellent sphenoids are found coating and sometimes even within the sphalerite specimens from Joplin, Mo.
These crystals may follow parallel lines of the sphalerite, possibly cleavage lines. The presence of these large chalcopyrite crystals (Fig.2), can perhaps be explained when we remember that successive additions of sphalerite might have covered up successive crusts of chalcopyrite. But even here, though the chalcopyrite sometimes is within the sphalerite, there are none of the characteristic feeding stringers found in the deposits of juvenile water origin.
While the occurrence of chalcopyrite in sphalerite seems to have been curiously persistent in the specimens examined by the writer, it is evident that there may be reasons for the absence of chalcopyrite in some cases.
These are:
(a) The total absence of copper in the ore-bearing solutions.
(b) Copper, though present, might have been precipitated before the introduction of the sphalerite. While this case is rare, since chalcopyrite nearly always postdates the sphalerite, the determination of the order of the two in any deposit is necessary.
(c) The copper may escape observation because the dots are so small.
(d) More than one specimen from a locality should be examined, to avoid overlooking any copper.
(e) Sphalerite, if secondary, might show the characteristics of a supergene mineral, although occurring in a primarily hypogene deposit.
(f) The sphalerite might occur in such small quantities that surrounding minerals may have prevented its attack by the chalcopyrite solutions.
Application of Principle.—It may seem a little rash to draw any definite conclusions from the examination of the limited number of occurrences covered by this paper; nevertheless, if the principle suggested is correct it might be possible to:
(a) Differentiate between a primary ore deposited by ascending or descending waters.
(b) Indicate the character of the solutions that had deposited an ore, now of metamorphosed character.
(c) Identify secondary from primary sphalerite in a deposit formed initially by ascending waters.
Galena-pyrrhotite Impregnations.—In a few specimens, minute dots of galena and pyrrhotite were found in the sphalerite, although in much smaller quantities than those of chalcopyrite, but in every case the deposits were of the deep-vein zone type. At Idaho Springs, some tetrahedrite dots have also been found in the sphalerite, but this seems rare.
Relative Isolation of the Sphalerite.—In direct contrast to the close association of chalcopyrite with sphalerite, and the replacement of the latter by the former, we see few other metallic minerals showing similar relationship.
Many writers have been led to believe, by assays of sphalerite ore with which only a small amount of galena was associated, that the silver, gold, antimony and similar metals found in the assay were contained as small grains in the sphalerite. Perhaps it is so, but magnifications up to 100 diameters have not disclosed them, while much less magnification has shown numerous compounds of these metals to be present in galena, and has also shown the prevalence of chalcopyrite, and in some cases pyrrhotite, in the sphalerite.
Reference to Table 1 shows how few elements were found in the sphalerite aside from copper and lead, and how little zinc was noted in other minerals.
Evidence furnished by Bastin, Wolcott and others shows that even in deposits characterized by silver minerals, these compounds show a distinct aversion to the sphalerite; further, the experiments of Bastin and Palmer have shown that sphalerite acted only weakly as a precipitant of gold from slightly acid AuCl2 solutions, while it was entirely inactive toward the silver, in a silver chloride solution.
F. C. Lincoln found that sphalerite was the fourth most important associate of gold in 585 veins which he examined megascopically. Of 163 occurrences of sphalerite in the same vein with gold, there seemed to be some relation in 69 cases. In 18 of these the gold was disseminated in the sphalerite, in five it was on the sphalerite, in seven intergrown with it, and in 37 with it.
Bastin found that polybasite, though often in the same section with sphalerite, and postdating it, preferred the quartz-galena contact. Moreover, the silver mineral replaced the galena but left the sphalerite untouched.
Similar relationships are described by Bastin from Clear Creek and Gilpin Counties.
At Butte, Mont., sphalerite may be often intimately associated with pyrite, bornite, chalcocite and galena, but rarely with enargite, and at Park City, Boutwell has commented on the non-association of sphalerite and tetrahedrite.
There seem, however, to be occasional exceptions, as shown by graphic intergrowths of sphalerite and jamesonite at Zimapan, Mex or of pearcite and sphalerite in Clear Creek County, Colorado. In this last named area, enargite, polyargyrite, and polybasite also replace the sphalerite. These unusual cases may be due to peculiar conditions of temperature and pressure.
Order of Crystallization.—As previously pointed out, in all the specimens examined, it seems to be almost universally true that sphalerite is the first valuable, and the second metallic sulphide to crystallize from ascending solutions. This fact has been noted by other writers for Butte, Mont.; Ducktown, Tenn.: Ely, Vt.; Capleton, Que.; Gordonsville, Va.; and Idaho Springs district, Colo., etc.
Great care must be exercised, of course, in studying ores for the purpose of determining the order of succession, but the following criteria seem to be fairly reliable: (a) Younger minerals may cut older ones in veinlets; (b) a younger mineral may fill cleavage cracks in the older; (c) a mineral following well-defined directions as dots or stringers in another is the younger; (d) a later mineral may occur along the contact of two others, and even replace one or both along its boundaries; (e) if possible, a large hand specimen should always be examined in connection with the polished pieces.
The mere surrounding of one mineral by another, as seen in the plane surface examined, is not necessarily definite proof that the inclusion is the older.
In the meteoric water deposits, different conditions prevail, and we might look for a different order of crystallization, provided all substances were present in the same solution. Galena might then be deposited first, followed by sphalerite. Siebenthal found that this is generally true in Missouri and Arkansas, but most of his examples were from vugs. But Bain, Grant, and Ulrich, in Wisconsin, Illinois, arid Kentucky, respectively, found the sphalerite often as the first product. However, they mention the recurrence of the sphalerite and galena in several stages, the evidence also being taken from vugs.
Microscopic evidence seems to be also at variance, for in some cases the youth of the galena is shown by its occurrence as veins in the sphalerite, elsewhere the sphalerite appears to be cutting the galena, or again contemporaneity of deposition seems to rule.
As there appears to be little chance for any great quantity of secondary galena to have formed, we cannot ascribe the varying relationships to this. Our only recourse is to, credit the varying observations and assume that as the deposition of the lead and zinc was, in some cases at least, more or less continuous, there may have been many overlapping generations, and hence varying associations.
Further, the relative amounts of lead and zinc in a solution would largely determine their order of deposition.
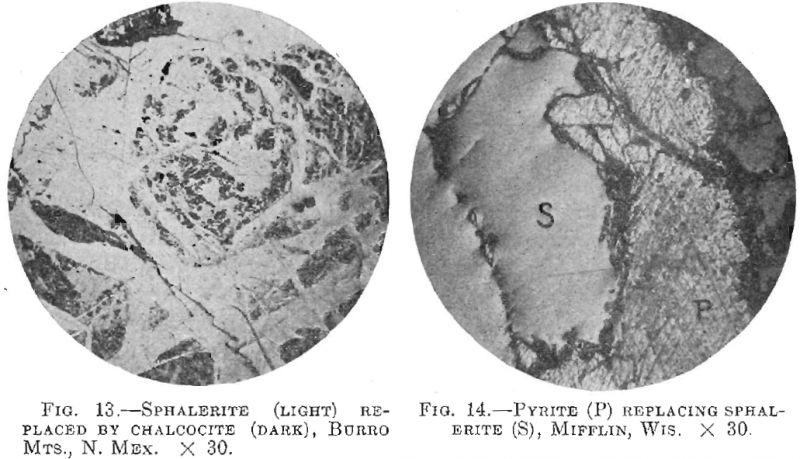
4. Sphalerite Replaced by Metallics.—The author found sphalerite replaced by galena, tetrahedrite, chalcopyrite, and pyrrhotite. It has also been found replaced by chalcocite (Fig. 13), covellite, and pearcite, bornite, stromeyerite, and ruby silver.
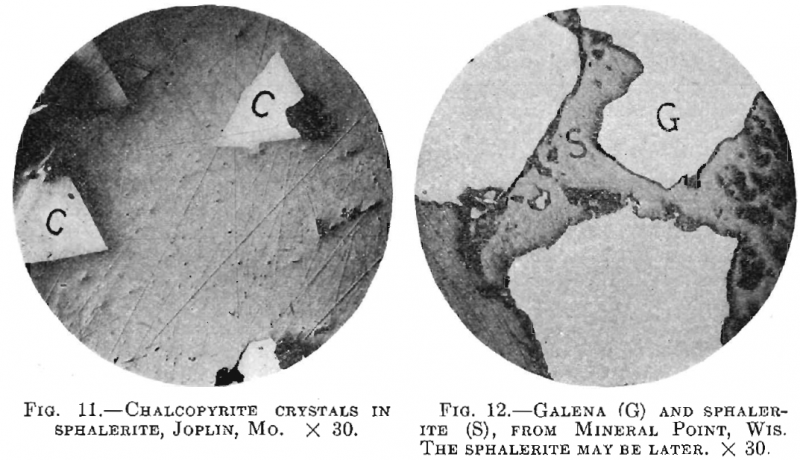
5. Sphalerite Replaced by Non-metallics—’The replacement of sulphides by non-metallics has been rarely noted hitherto, although one undoubted case has been described. In this work one analogous case has come under the writer’s notice in which quartz appears to be replacing sphalerite (Fig. 12).
CONCLUSIONS
The foregoing facts seem to warrant the following conclusions:
Sphalerite in General.—1. Silver compounds were not noted in any of the specimens of sphalerite, and observations by others indicate that they are rare.
2. Sphalerite may replace calcite, dolomite, quartz, pyrite, marcasite, hematite, and pyrrhotite; it may be replaced by galena, tetrahedrite, chalcopyrite, chalcocite, bornite, pearcite, stromeyerite, and ruby silver.
Sphalerite from Meteoric Waters.—3. This type was marked by complete absence of chalcopyrite dots in the sphalerite.
4. No uniform order of deposition of sphalerite, galena, and marcasite was noted.
Sphalerite of Hypogene Origin.—5. Chalcopyrite dots and stringers are generally associated with the sphalerite. They are most numerous in contact-metamorphic deposits.
6. Sphalerite in the occurrences examined is with one exception the first valuable, and second metallic sulphide deposited.
In conclusion, the writer wishes to express his appreciation to Prof. H. Ries and Prof. R. E. Somers, for suggestions and criticisms made during the course of the work.
DISCUSSION
THOMAS L. WATSON, Charlottesville, Va. (written discussion).— Sphalerite, as is well known, occurs not only as a common constituent of many types of ore deposits formed under widely varying geologic conditions, but its sulphide mineral associates comprise a goodly number of species. In the investigation by Mr. Teas, data were obtained from a mineralographic study of about 200 ore specimens from 43 different localities grouped according to their genesis as follow;-: (1) Contact-metamorphic deposits, (2) deep-vein zone deposits,. (3) intermediate-vein zone deposits, (4) shallow-vein zone deposits, (5) meteoric water deposits. The object of the study was to determine the relations of sphalerite to the associated sulphide minerals and their possible genetic significance.
As a result of the investigation, Mr. Teas has deduced several important conclusions. Probably the one of largest interest and value was that sphalerite formed from magmatic waters, including contact-metamorphic deposits, could be distinguished, for the cases investigated at least, from sphalerite formed from meteoric waters, by development in the former of minute triangular or rectangular dots or stringers of chalcopyrite. Although chalcopyrite inclusions in sphalerite had been noted by previous observers, the probable significance of their apparent general occurrence in sphalerite of certain types of ore deposits and their absence from others was unknown.
The large number of ore specimens studied from more than 40 different localities in the United States and foreign countries, representing the principal genetic types of sphalerite-bearing deposits, gives considerable weight to the conclusions reached by the author. Should the conclusion drawn by Mr. Teas become generally applicable, a laboratory criterion of much value will have been established for distinguishing between sphalerite formed from juvenile and that from meteoric waters.
The principle seems applicable not only to sulphide deposits in which sphalerite is the principal constituent but to those in which it occurs as a very subordinate one, as shown by the study of ore specimens from one of the principal pyrite mines in Virginia. It may be possible also that the principle will have important application in differentiating primary from secondary sphalerite occurring in the same deposit formed from magmatic waters, although there have been comparatively few well- authenticated cases of secondary deposition of sphalerite. Furthermore, the records show the occurrence of secondary sphalerite in rocks other than limestones to be rare. Nevertheless, it would be of interest and possibly productive of results to extend the investigation into this phase of sphalerite genesis.
Sphalerite is a frequent constituent in many of the sulphide deposits of the southeast Atlantic States, and in some, especially in those of Virginia and Tennessee, it is the dominant sulphide mineral. Genetically, several different types of deposits are represented, which for purposes of this discussion may be broadly grouped into (1) those formed from meteoric waters, and (2) those formed either directly or indirectly from juvenile waters. The zinc deposits of the east Tennessee valley of Virginia and Tennessee are usually regarded as having formed from meteoric waters, while those deposits occurring in the metamorphic crystalline rocks east of the Blue Ridge in Virginia owe their origin to magmatic waters. Specimens of ores from each of these types of sphalerite-bearing deposits in Virginia were included in Mr. Teas’ investigation, with the result that the sphalerite of the two groups was differentiated on the basis of the principle stated, which confirms our knowledge previously gained from the facts of field occurrence, etc.
Mr. Teas has made an important contribution to our knowledge of sphalerite-bearing ore deposits, and it is to be hoped that the investigation will be continued in order to test the reliability and value of the conclusion as a safe laboratory criterion for differentiating between ore deposits containing sphalerite formed by magmatic waters and those deposited by meteoric waters.
L. C. GRATON, Cambridge, Mass. (written discussion).—The method of arriving at conclusions in regard to ores and ore deposition through the microscopic study of a suite of specimens, ordinarily collected by someone else, more or less representative oh the occurrences from which they come, labeled carefully or carelessly, completely or incompletely, as the case may be, is a method that possesses obvious dangers. It is an attractive method, and it is a feasible method for gaining both experience and geological results, particularly by the younger men in our universities. It is farthest from my intention to discourage this practice or to minimize the value of conclusions that may be reached by its means. I wish only to point out that such conclusions must necessarily be regarded as resting on somewhat incomplete or possibly sometimes erroneous foundations and therefore must be accepted and used with a certain reserve and caution. In general, the reliability of results attained by this method is likely to vary in direct proportion to the individual investigator’s personal experience with the field relations, that is to say, the broad geological relations, of the type of deposit under examination.
Mr. Teas has produced by the use of this method what seems to me a very good paper. It is gratifying to find that a number of conclusions which my associates and I have reached from the field and laboratory study of sphalerite-bearing copper ores find support in the results of his more intensive study of sphalerite in what are mainly zinc ores. While some of his conclusions may eventually suffer modification or even reversal, as happens, indeed, to the work of nearly all of us, his survey of this field and his summary of its features and its significance cannot fail to be helpful as well as an incentive to further study in this direction.
In one particular I venture to point out what seems to me an unfounded deduction and a possible illustration of the dangers that may attend the “specimen” method of investigation. I refer to the implication that the origin of a sphalerite-bearing ore, viz., whether of meteoric origin or produced by ascending solutions of deep-seated source, is indicated by the absence or presence, respectively, of small inclusions of chalcopyrite fin the zinc sulphide. Mr. Teas studies specimens from a number of occurrences which, upon whatever independent evidence is available to him, he concludes were formed by meteoric waters; in these he finds certain characteristics in the sphalerite. He also studies specimens from a number of other deposits which he or others have concluded were produced by ascending, juvenile waters; and in the sphalerite of these he finds other characteristics.
If the original distinction assumed as to origin is correct and if, as the discussion by Dr. Watson has noted, Mr. Teas’ finding of chalcopyrite in one case and none in the other, proves to be borne out by further evidence and study, then he certainly has afforded us a valuable and simple means of distinguishing between meteoric origin and magmatic origin in any new case which contains sphalerite that may come to attention. But if it should turn out that the group of deposits which he has thought are of meteoric origin are actually not of meteoric origin, or those which he has regarded as of juvenile origin are not to be so classed, then this difference between absence of chalcopyrite in one case and its presence in the other certainly does not mean that one group of deposits is of meteoric and the other is of magmatic origin. In other words, the conclusion is no more compelling or convincing than the initial premise on which it rests. And the observed difference with regard to chalcopyrite is simply one evidence of certain differences in mode of deposition of the two classes of deposits; it does not necessarily and inherently mean the difference between magmatic and meteoric solutions any more than it necessarily and inherently means the difference between acid and alkaline, or weak and concentrated solutions. One thing which it pretty evidently does mean, however, is that in one case, copper was available in the solution whereas in the other case probably little or no copper was available. And perhaps that is the only thing it does mean.
In connection with this subject it may be added that in certain instances that have come under my observation where the central part of a district or of a single orebody yields ores worked for copper, the accompanying black, iron-rich sphalerite commonly carries included particles of chalcopyrite; but on the outlying edges of the district or deposit, where copper values are lower or wanting, the sphalerite may be lighter in color because lower in iron content, and may be quite free of chalcopyrite inclusions. Yet no one, I believe, could successfully maintain that the ores in the two situations differ essentially in origin.
H. Ries, Ithaca, N. Y. (written discussion).—The permanent establishment of any criterion will, of course, depend on whether the fundamental facts on which it is based are correct, and no theory can be accepted as the true one until it has stood the test of searching criticism. It seems to me that any one who reads Mr. Teas’ paper carefully will see that he has assumed a somewhat conservative attitude. The results which he has presented are based upon a somewhat extensive series of specimens, collected in nearly every case by persons of known responsibility, and the laboratory study consisted not merely of an examination of polished chips, but of all the hand specimens, as well as the literature bearing on the deposits considered. The origin of the different deposits had to be determined in practically every case from the statements of reliable authorities, and it is perfectly safe to say, as Mr. Graton does, that we may find some of these genetic classifications to be incorrect, but, for that matter, the same might apply to some of the determinations of mineralographers.
I believe, however, that Mr. Teas was warranted in assuming that most of the determinations of origin were probably correct, and that the observed phenomena were sufficiently persistent to warrant placing them on record. He recognizes, however, that the suggestion which he advances may require further corroboration, for he says: “It is not supposed that the investigation of such a limited number of occurrences may warrant conclusive and universal generalizations, but the conclusions reached are believed to be reliable so far as the specimens that were examined are concerned.”
On a later page, he remarks: “It may be a little rash to draw any definite conclusions from the examination of the limited number of occurrences covered by this paper; nevertheless, if the principle suggested is correct it might be possible to draw the following conclusions,” etc.
The fact therefore remains, that in deposits usually regarded as of magmatic origin, the sphalerite so often shows these curious chalcopyrite inclusions, while none were observed in sphalerite usually regarded as being deposited by meteoric waters.
Discussion:
The study of mineralography is a most attractive one, but it is, of course, one that should be pursued with caution. In many cases, it is an indispensable aid to field study, but this is not intended to say that it cannot be used for the solution of problems in the laboratory independent of field work, for I believe it can. One danger, of course, lies in attempting to draw conclusions from one or two polished chips alone. This is sometimes safe, where the evidence is exceedingly clear; at other times it is not, and so the investigator must use very careful judgment. I must confess that some of the published illustrations of polished ore surfaces do not, in my opinion, always show what the authors have claimed for them.
The Relation of Sphalerite to Other Sulphides in Ores by L. P. Teas, M. A. Ithaca, N. Y.
