In flotation, pH control is a vital method to control selective mineral separation. A standard depressant that is used is an Alkali. An Alkali is any substance that when mixed with Acid will neutralize each other and form a new chemical substance called a BASE. This is very important. The ore may be either alkaline or Acidic. To have a positive control over flotation, it is necessary to be able to determine which and to what degree it is. A cheap way to take this measurement, LITMUS PAPER or a pH METER is used.
This is a very important measurement for the flotation operator. Some minerals like Iron pyrite will not float well in an alkaline flotation circuit. An alkaline solution is also slightly caustic, this will help clean the surface of the mineral. This strengthens the bond between the collector and the surface of each mineral particle. These two characteristics make alkaline solutions both a depressant and a conditioner. If you are in a mine that is using cyanide for one reason or another an alkaline circuit is a must. Cyanide and acid is the chemical combination that produces the gas that is used for the execution of condemned prisoners. When the two are mixed together the gas that is formed is tasteless, odorless, and colorless. It is also deadly. A worker that is working around cyanide may be poisoned by ingestion that means eating it, breathing it as a gas, or absorbing it through his or her skin.
To control the Alkalinity of the ore some of the reagents that may be used are SLAKED LIME, QUICK LIME, CAUSTIC SODA, and SODA ASH. This group of reagents aren’t the only depressants there are, but as they are important for other usages I thought that I would give them special mention.
The job of depressants as a whole could be summed up as to interfere with the reaction between selected minerals and the other reagents within the recovery process. Like all the reagents, depressants are only selective and effective within limited volume range. Too little reagent and the unwanted mineral will begin to float, too much reagent and the wanted mineral will begin to be depressed.
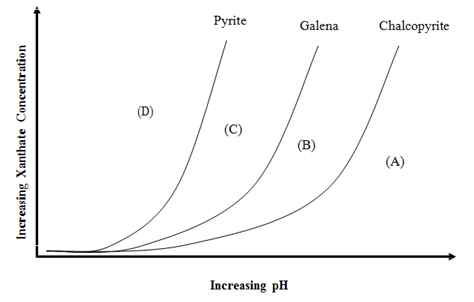
The simplest modifiers are pH control chemicals. The surface chemistry of most minerals is affected by the pH. For example, in general minerals develop a positive surface charge under acidic conditions and a negative charge under alkaline conditions. Since each mineral changes from negatively-charged to positively-charged at some particular pH, it is possible to manipulate the attraction of collectors to their surfaces by pH adjustment. There are also other, more complex effects due to pH that change the way that particular collectors adsorb on mineral surfaces.
pH Modifiers
The function of modifying reagents, as has already been explained, is to affect the surfaces of the gangue particles in such a way as to render them less floatable than they otherwise would be.
By far the most commonly used are lime and soda ash (sodium carbonate). It should be realized, however, that before either of them can have any effect on the surfaces of the gangue particles, they first neutralize the acidity and precipitate the dissolved salts that may be present in the water of the pulp. Pyritic ores, especially those containing pyrrhotite and marcasite, are likely to give rise to considerable acidity after grinding and to the presence of ferrous and ferric salts ; some ores also contain the soluble sulphates of calcium and magnesium which may cause interference, whilst calcium sulphate is often brought into the circuit in the water supply. The flotation of the minerals may be affected by the presence of one or more of these compounds to the extent that they must be neutralized or precipitated before the promoting reagent will function properly.
It is usually necessary to add soda ash if the interference is caused by calcium sulphate, but the cheapest way to precipitate any other salts that are present and to neutralize acidity is by means of lime. Its addition is controlled in practice by frequent titration of samples of water filtered from the pulp in order to determine its alkalinity ; this is maintained at a predetermined figure high enough to ensure that neutralization and precipitation are complete. Ordinary titration for alkalinity with an indicator such as methyl-orange is often satisfactory, but the more accurate pH determination is becoming increasingly common.
After neutralization and precipitation has been accomplished, any excess of lime or soda ash will be available for modifying the faces of the gangue particles. The action of soda ash is that of a deflocculator ; it makes the gangue particles more readily wetted by water and so decreases their floatability. It is rather doubtful if lime acts in the same way, since its tendency is rather to flocculate the gangue. It also has a positive action in depressing metallic sulphides and is employed more for the purpose of preventing the flotation of unwanted minerals than for its effect in sinking the gangue; as such it is more a depressing than a modifying reagent. Whatever the physical or chemical action of these alkalis, it is generally found that they have to be added in sufficient quantity to maintain the alkalinity of the pulp at a critical figure which has been found by experience to give the best flotation results. This may be only slightly in excess of, or even below, that of the incoming water, in which case the reagent is probably merely neutralizing acidity and precipitating dissolved salts ; if it is much in excess of that of the water supply the inference is that the alkali is functioning as a modifying reagent. The neutral point in the pH scale is 7.0, and the alkalinity of a pulp normally ranges from 7.0 to 8.5, although there are cases where a pH value as high as 13.0 has to be maintained.
Soda ash is added to the circuit as a solid ; the addition of lime is sometimes made in this way, but it is generally preferable to feed it to the circuit in the form of milk of lime. The consumption of either reagent may be anything up to 6 lb. per ton of ore. Soda ash is seldom added in excess of this amount, but lime is sometimes needed in greater quantity.
Cement is occasionally substituted for lime, and other alkalis such as caustic soda find a limited application at times in place of soda ash.
Sodium silicate is employed as a deflocculator of gangue slime in cases where the latter is too floatable to be kept out of the concentrate by any other means. Its application is confined mainly to the flotation of lead and zinc ores and tailings. Some inorganic salts decompose it and it is therefore often necessary to precipitate them by means of soda ash before the silicate is added. In any event its deflocculating action is generally most effective at a definite critical alkalinity, which has to be determined for every ore. The disadvantage of sodium silicate is that it disperses the gangue slime so thoroughly, if much of it is used, that it is impossible to settle the fine part of the tailing subsequently in thickeners or a tailing dam. Lime or some other flocculating reagent can be added to coagulate the slime but the amount needed is often prohibitive. Consequently it is not usual to add more than 1 lb. of sodium silicate per ton of ore, even though the addition of a larger amount might improve the grade of concentrate in the flotation section.
In spite of the fact that “ water glass ” contains only about 50% of sodium silicate (the rest being water) it is the variety that is generally employed for flotation purposes since it can be readily dissolved in hot water. It is usually made up into a solution containing 30 to 50% of “ water glass Solid silicate with little or no combined water can be obtained in lump form, but is seldom employed because an autoclave is necessary to dissolve it on account of the fact that its solution is only possible at a temperature well above the boiling point of water.
Starch is often more effective than sodium silicate especially with ores containing gangue minerals such as talc, kaolin, serpentine, etc., which have a strong tendency to float. The addition of a small amount of starch—generally 0.2 and seldom more than 0.5 lb. per ton—will usually deflocculate such minerals completely, but care is required in its use as excess may depress the valuable minerals as well. Starch is particularly useful in the flotation of native gold in the presence of flocculent gangue slime ; a small amount of the reagent will generally deflocculate the gangue without affecting the gold. Sodium silicate used for a similar purpose has a strong tendency to depress the gold.
So called “ soluble starch ” is too expensive a reagent to use for commercial purposes, and ordinary starch is only partially soluble in water even after prolonged boiling. The best method of preparing a solution is to make ordinary starch up into a paste with cold water and then boil it with a dilute solution of sodium hydroxide. A 5% starch solution can be made in this way by using a 2% solution of sodium hydroxide.
Sulphuric acid is employed occasionally as a modifying reagent, but its use is steadily decreasing, being confined chiefly to the treatment of lead and zinc ores. There are, however, one or two ores containing sulphide copper minerals which need an acid circuit for successful flotation.