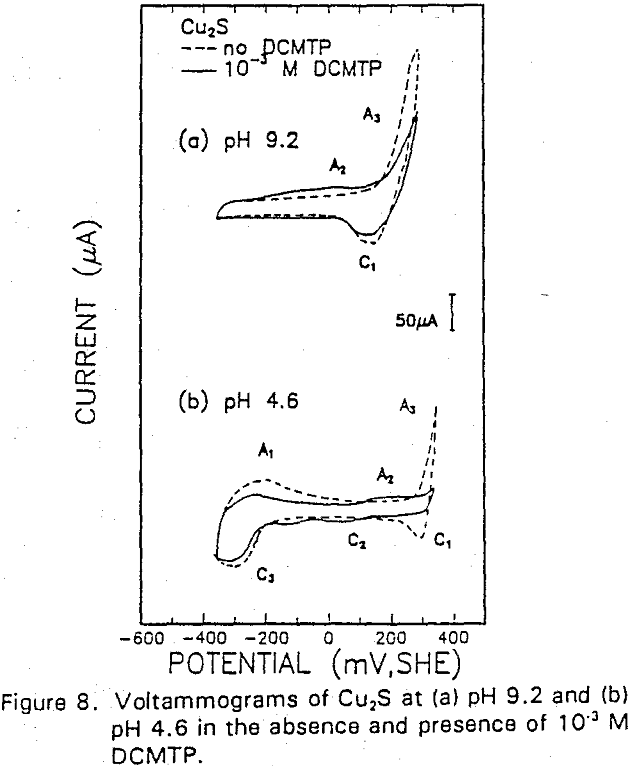
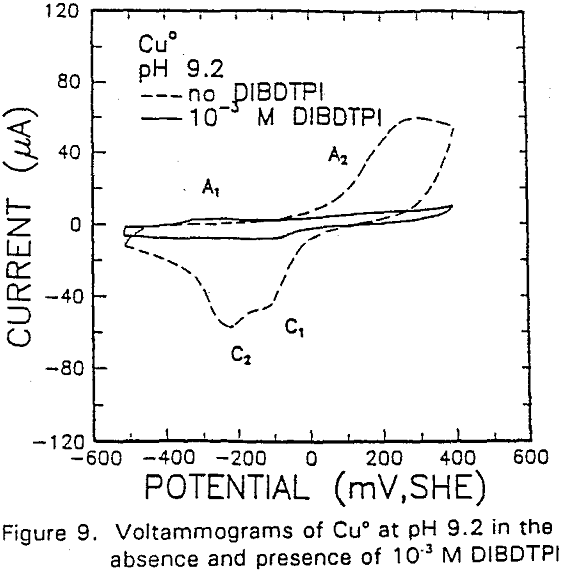
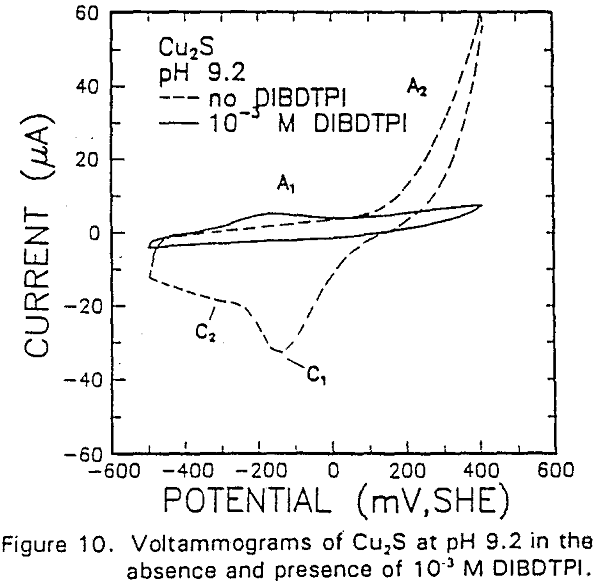
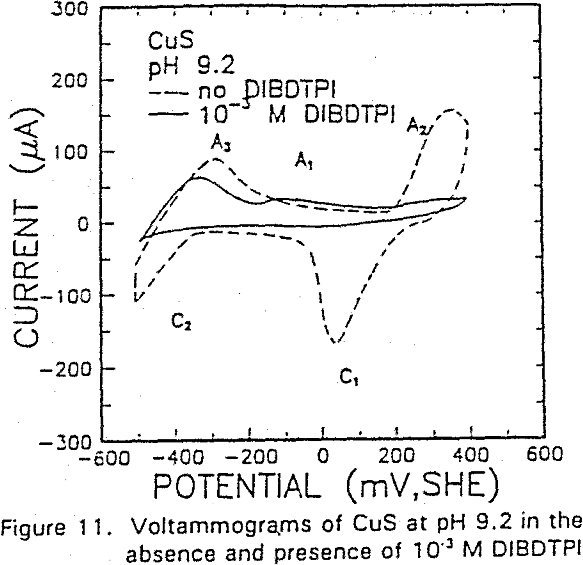
The formation of metal xanthate can be considered to comprise an initial electrochemical reaction (E),
MS → Mn+ + nS° + ne……………………………………………………………[1]
followed by a chemical reaction (C),
Mn+ + nX- → MXn………………………………………………………………..[2]
Thus, the xanthate adsorption mechanism is controlled by both Eh and pK, and consequently, one can expect that the same may be applied to the adsorption of modified thiol-type collectors, such as thionocarbamates, thioureas, monothiophosphates and dithiophosphinates. One major difference between these collectors and xanthates is the ease with which xanthates are oxidized. The oxidation potential of the xanthate/dixanthogen couple is -0.06 V at 10 -7 M potassium ethyl xanthate, which is well within the range of potentials encountered in operating flotation pulps.
Experimental
All of the sulfide minerals were acquired from Ward’s Natural Science Establishment. The modified thiol-type collectors (>95% pure) were from the American Cyanamid Company. The structure of the collectors studied here are given in Table I. The voltammetry experiments were conducted in 0.05 M Na2B4O7 (pH 9.2) and 0.5 M CH3COOH/- 0.5 M NaCH3COO solutions (pH 4.6).
Results and Discussion
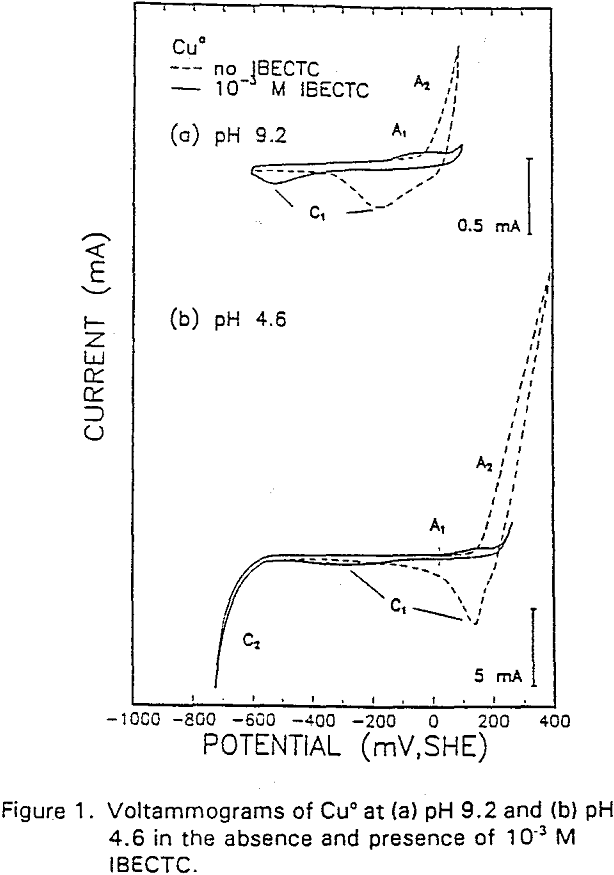
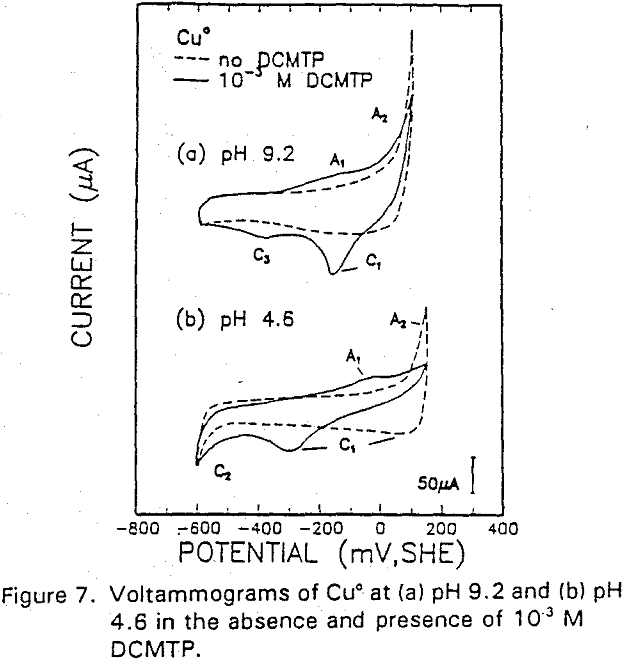
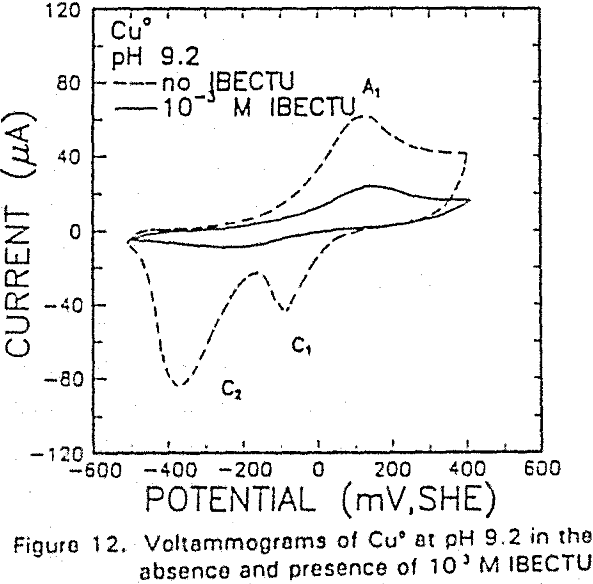
The potential was cycled between -750 and 300 mv at a scan rate of 50 mV/sec. The voltammogram obtained in the absence of IBECTC shows the oxidation of Cu° to Cu20 (A2), which appears above 120 mV. On the return scan, cathodic peak C1, which is due to the reverse of A2, is observed near 100 mv.
Cu° + IBECTC → Cu(IBECTC’) + H+ + e……………………………………….[3]
Cu° → Cu+ + e……………………………………………………………..[4]
Cu+ + IBECTC → Cu(IBECTC)’ + H+…………………………….[5]
The stability of Cu+ ions produced in Reaction [4] may be attributed to the presence of IBECTC, which has been found to form a complex with Cu+ but not with Cu²+. Studies of Cu° oxidation using ring-disc electrodes in borate buffer solutions have also established that cuprous ions are formed as an intermediate with a measurable lifetime.
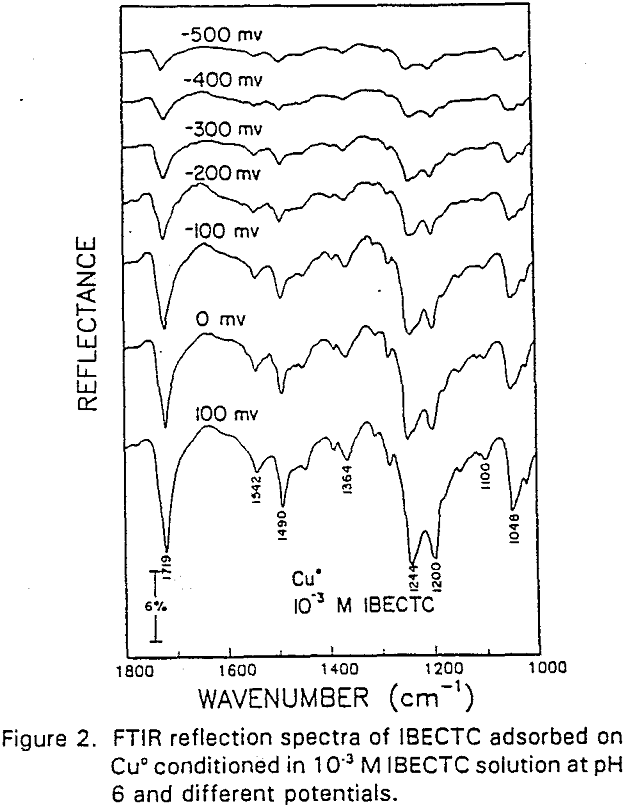
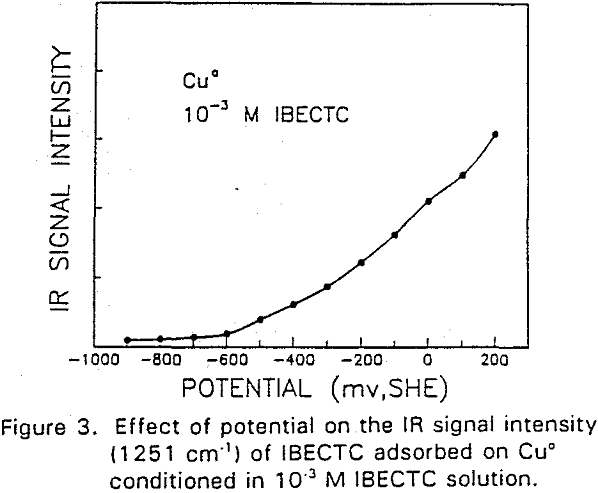
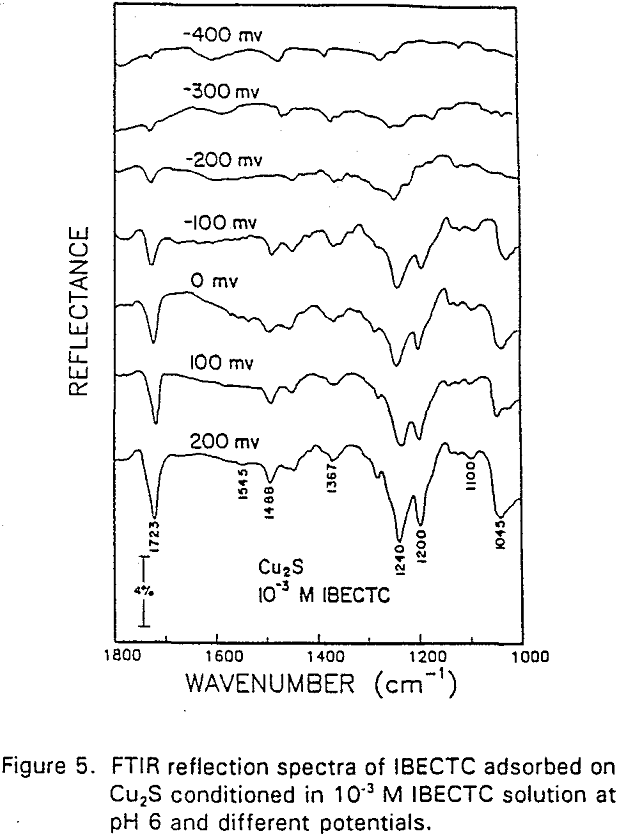
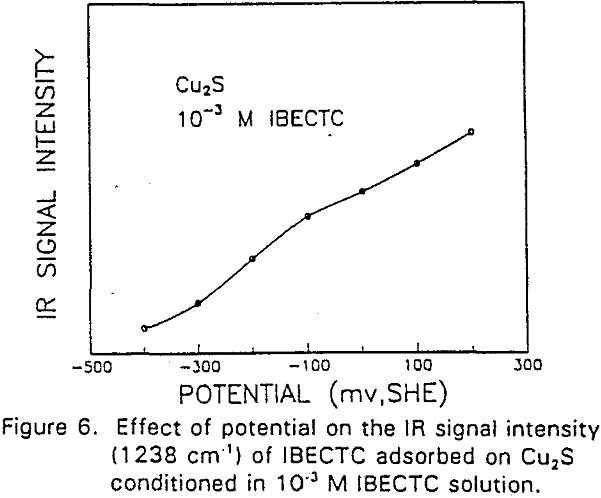
IBECTC adsorption on copper becomes significant at a potential of -500 mv, which is much lower than where the IBECTC interaction peak is observed in the voltammogram. This is probably due to the high pK value of the Cu-IBECTC compound, thus, requiring only a small amount of copper ions to form the thionocarbamate compound. Thermodynamic calculations (Youze ?) showed that very small amounts of copper ions are present in solution at such low potentials. Increasing the potential does not result in any distinct shift or appearance of new peaks in the spectrum, even at potentials where copper starts to oxidize.
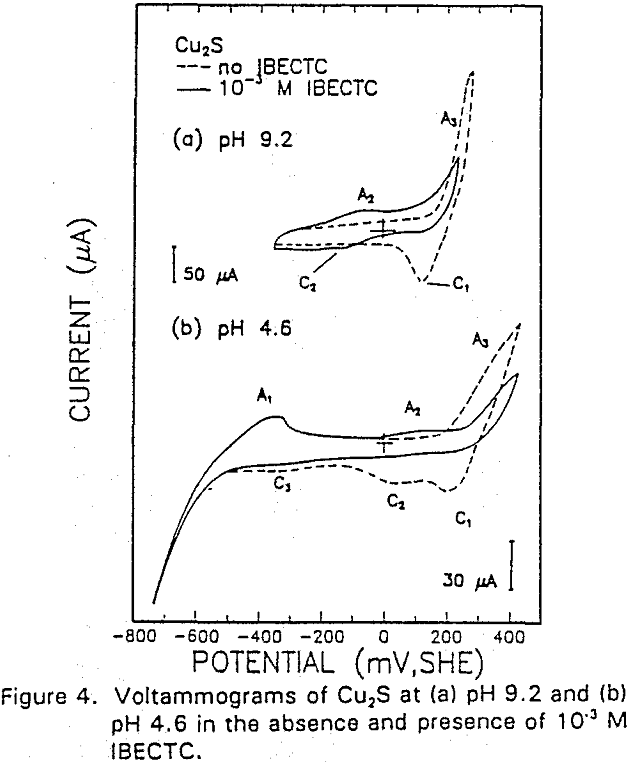
The reverse of this reaction is shown by the cathodic peak at C1. The additional cathodic and anodic peaks, C4 and A3, occurring at lower potentials and acidic conditions are not observed here due to the higher cathodic limit used in this voltammogram.
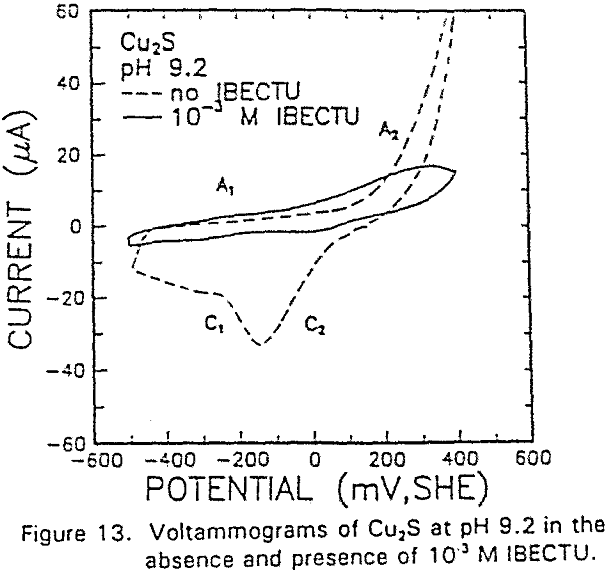
The interaction of IBECTC with chalcocite is shown by A1, and may be attributed to reaction (10). This peak has been shifted to a lower potential, which would support the pH dependent EC reaction. Due to the IBECTC adsorption, the oxidation of chalcocite is observed to be inhibited.
The adsorption of IBECTC is found even at the starting potential of -400 mv. An increase in potential does not seem to cause any spectral shift or appearance of new peaks. This indicates that no new adsorption products were formed at the potential where the IBECTC interaction peak is observed in the voltammogram.
The voltammograms for copper and copper sulfide electrodes in the presence of the modified thiol-type collectors showed an anodic peak that may be attributable to collector interaction. This interaction may be represented by a coupled chemical reaction of the EC type involving the oxidation of Cu° or Cu2S, followed immediately by the formation of Cu-collector compound.