Table of Contents
A part of the U.S. Department of the Interior, Bureau of Mines, research program is to develop improved or alternative methods for metal recovery from domestic resources, and thereby help to insure an adequate supply of mineral raw materials for the U.S. economy. Since about 80 pct of the nickel requirements are imported, research studies have been initiated to develop domestic resources. The largest domestic nickel sulfide deposit (referred to as the Duluth Complex) is located in northeastern Minnesota between Duluth and the Canadian border. This deposit has been estimated to contain about 5 million tons of nickel and about 14 million tons of copper at a cutoff grade of 0.5 pct. Some exploratory mining is presently being conducted on the deposit, but only limited metal extraction data are available.
The steps utilized to extract nickel and copper from sulfide ores usually involve flotation, smelting, and leaching. To aid in the separation of nickel and copper, the leaching step is usually conducted in several stages. Presently, considerable attention has been given to a two-stage leaching process that has been used on matte from Africa containing 39.5 pct Cu, 40.0 pet Ni, 0.2 pet Fe, and 16 pct S. The Duluth Complex matte (61.2 pet Cu, 9.7 pet Ni, 3.3 pet Fe, and 21 pct S) made at this laboratory had a higher copper-to-nickel ratio and a higher percentage of sulfur and iron. The high iron content in the Duluth Complex matte is necessary to obtain over 70 pct Ni recovery in the smelting operation.
Since it was expected that mattes with different compositions leach differently, research was initiated on a two-stage matte leach (fig. 1) similar to that described by Llanos, Queneau, and Rickard. With this method, a portion of the nickel is preferentially extracted from the matte in a first-stage atmospheric pressure leach which results in a solution containing a substantial quantity of nickel and essentially no copper, iron, and acid.
The matte residue from the first-stage leach is digested in a second-stage autoclave to extract the residual nickel and all the copper. Iron is also dissolved and is removed before copper electrowinning. The electrowinning
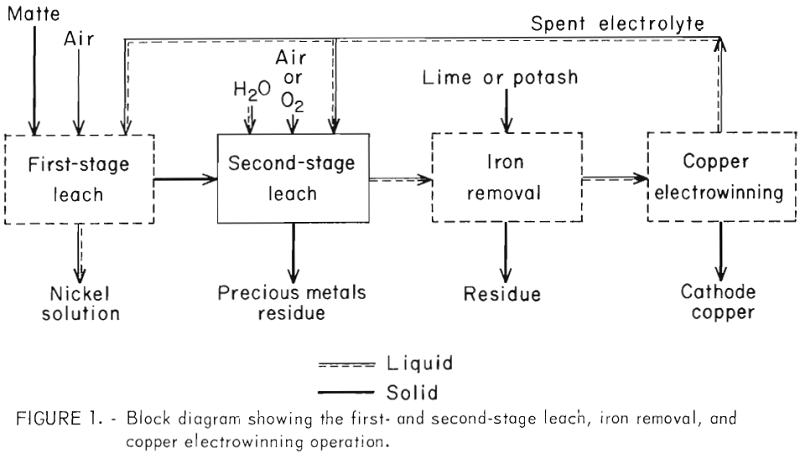
step generates acid which is recycled to the first and second leaching operations. Several investigators have published on the atmospheric pressure leach of matte but essentially no experimental details are available on the autoclave step.
Numerous lixiviants have been used to leach metal sulfides in autoclaves. An ammoniacal solution has been used for two decades by Sherritt-Gordon on a commercial scale to recover nickel, copper, and cobalt from sulfide feeds. Mackiw and Kunda have reported the use of acid for mattes that contain precious metals. (The precious metals content of the matte used in this research was more than 5 oz/ton.) Acidic solution of chorides, or chorine under pressure, have been used and some of the problems with these lixiviants have been reported by Tegg. Nitric acid and nitrates are costly, their reactions with sulfides produce various nitrogen oxides, and the regeneration of the lixiviant is difficult. Sulfuric acid, on the other hand, is a byproduct of most sulfide leaching operations and has the advantage of introducing no extra anions into the solution. High-pressure sulfuric acid processes have been used on a commercial scale in the U.S.S.R., Africa, and Cuba, but very little operating data are available.
Only very limited data have been disseminated on sulfuric acid leaching in autoclaves of matte and matte residues; therefore, tests were initiated to achieve essentially complete extraction of the nickel and copper. The initial tests were conducted on matte because of its availability and to compare its leaching characteristics with those of the matte residue.
Materials
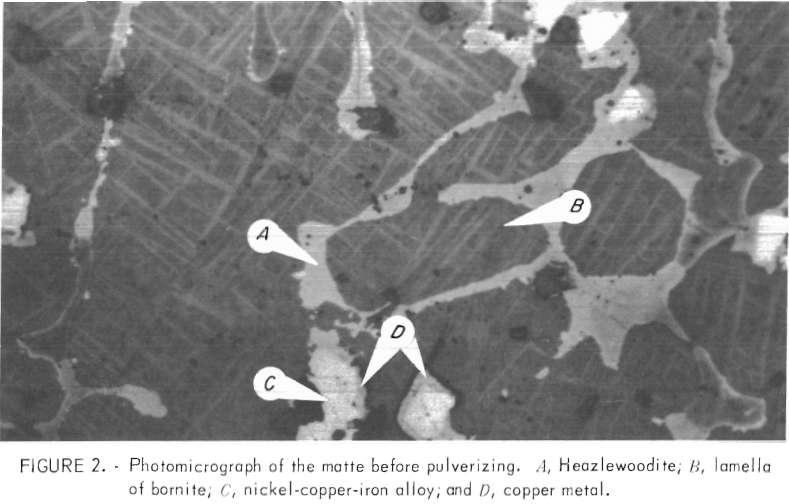
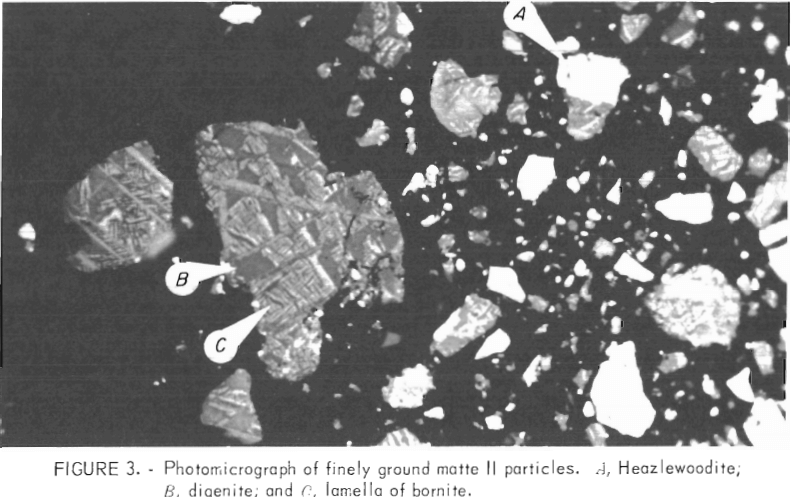
The photomicrographs of the matte before and after grinding are shown in figures 2-3, respectively. X-ray and microscopic examination of the matte indicated the phases were digenite (Cu1.8S), bornite (Cu5FeS4), nickel-copper- iron alloy, and metallic copper. The off-white colored grains (marker A, figs. 2-3) are heazlewoodite. The light-colored lamella in figure 3 are bornite (marker C) dispersed in a matrix of digenite (marker B). Marker D in figure 2 depicts the presence of small quantities (0.1 pct) of metallic copper.
The matte grinding procedure involved placing 275 grams of minus 4-mesh (standard Tyler screen) matte and 225 ml water in a rodmill (7 inches diameter and 9 inches long) containing 26 stainless steel rods (¾ inches diameter). The mill was rotated at 76 rpm for 15 min. After several pounds were ground, the batches were blended and referred to as matte I. Matte was also ground for 20 min and this composite was referred at matte II. The percentages of minus 325 mesh in mattes I and II were 90 and greater than 98 pct, respectively.
A matte residue composite was obtained by combining several first-stage residues that were produced by digesting 1 kg of matte II with 1 liter of spent copper electrolyte (50 g/l H2SO4, 20 g/l Cu, 30 g/l Ni) for 7 hours at atmospheric pressure and 70° C. The residues were dried at 110° C and passed
through a 65-mesh screen. The chemical analyses of matte and the matte residue are shown in table 1. From these atmospheric pressure leach results, it appears that copper was cemented out of the solution and 20 pet of the nickel was extracted from the matte. The nickel content of the matte residue decreased by about 2 pct and the copper increased by about 1 pct. Microscopic examination showed no apparent change by the atmospheric pressure leach; however, the X-ray diffraction patterns indicated that of the peak heights of Ni3S2 decreased and the NiS increased.
Experimental Procedure
The tests were conducted in a 4-liter stainless steel (type 316) autoclave equipped with a Pyrex reaction vessel and titanium sampling tubes, 5-blade turbine impeller, and cooling coil similar to that described by Leak. A titanium shutoff valve was located between the autoclave vent pipe and an ice-cooled 4-liter steam trap. The pressure was held constant via an inlet and an exhaust gas regulator. The gas was injected above the liquid level, but the operation of the agitator at 700 rpm helped to transfer the gas to the slurry. The airflow rate was maintained at 1.0 1/min (STP). With pure oxygen, no gas was vented and only sufficient oxygen was added to maintain a fixed pressure. In the oxygen tests, the autoclave was evacuated and back-filled three times to remove the residual nitrogen.
The procedure for initiating an experiment involved placing 75 grams of solid into the reaction vessel and installing the autoclave cover. The lixiviant (1.5 liters) was added through the filling port and the agitator, gas flowmeter, and heater were turned on. The desired operating temperature and pressure were reached in about 1 hour, and held for 6 hours. The slurry was then cooled to less than 100° C in about 2 min. About 15 hours later, the cold slurry was re-pulped and boiled at atmospheric pressure for 1 hour to redissolve all the nickel and copper sulfate crystals. The hot slurry was filtered and the residue washed at a wash-to-residue volume ratio of about 10. The wash water was boiled down to almost dryness and combined with the liquor. The liquor and the residue were analyzed, and the average percent metal extraction was calculated. The calculated material balances indicated that the charge and discharge values were within ±5 pct. When the weight loss was more than 96 pct, the residue was not analyzed for base metals, and only the liquor analyses were used to determine the percent extraction. This was also the case in the agitator speed studies where 5-ml liquid samples were taken during the test at specified intervals.
Results and Discussion
The autoclave leaching results are divided and discussed under the following topics: Leaching characteristics of matte, leaching behavior of matte residue, liquor studies, and residue data.
Leaching Characteristics of Matte
Influence of Pressure, Temperature, and Lixiviant Composition
The initial tests were conducted with finely ground matte II and 100 g/l H2SO4 made from reagent-grade sulfuric acid. The results in tests 1-4 (table 2) indicated that 95 pct of the iron and nickel were extracted when the autoclave was heated to 180° C and pressurized with air to 250 psig, but this level of copper extraction was not obtained until the pressure was increased to 400 psig. Increasing the temperature from 180° to 190° C resulted in extracting 95 pct of the copper at a lower pressure (350 psig). The residues were analyzed and the main constituents were sulfur (about 10 pct was in the elemental form) and copper. X-ray diffraction of these residues indicated that the predominant copper mineral was covellite (CuS). These results indicated that copper sulfide was more difficult to dissolve than the nickel and iron sulfides.
The influence of the lixiviant composition was determined by changing from the 100 g/l H2SO4 to a spent copper electrolyte containing 60 g/l H2SO4, 20 g/l Cu, and 30 g/l Ni. This acidity decrease (tests 4 and 7) resulted in no major change in the extraction; however, when the H2SO4 concentration was further decreased to 50 g/l (while retaining the same copper and nickel content), the iron content in the residue increased and a reddish-brown residue was obtained (test 8). X-ray diffraction analyses of this residue indicated that iron was precipitated as hematite (Fe2O3).
Agitator Speed and Leaching Time Studies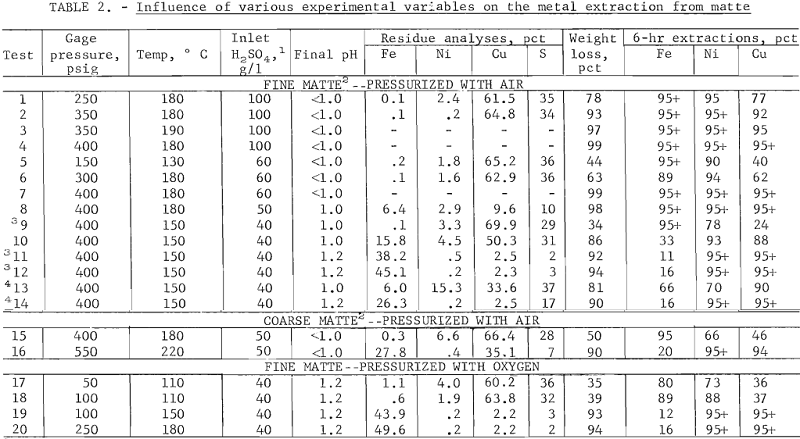
The influence of the agitator speed was investigated at 150° C with a spent copper electrolyte containing 40 g/l H2SO4 because at these conditions, incomplete extraction was expected at 1,700 rpm. However, at 1,300 and 1,700 rpm, over 95 pct of the copper and nickel (tests 11-12) was extracted, but only 88 and 93 pct of the copper and nickel, respectively, were extracted at 700 rpm (test 10). At 100 rpm (test 9), the copper extraction further decreased to 24 pct but the iron extraction increased.
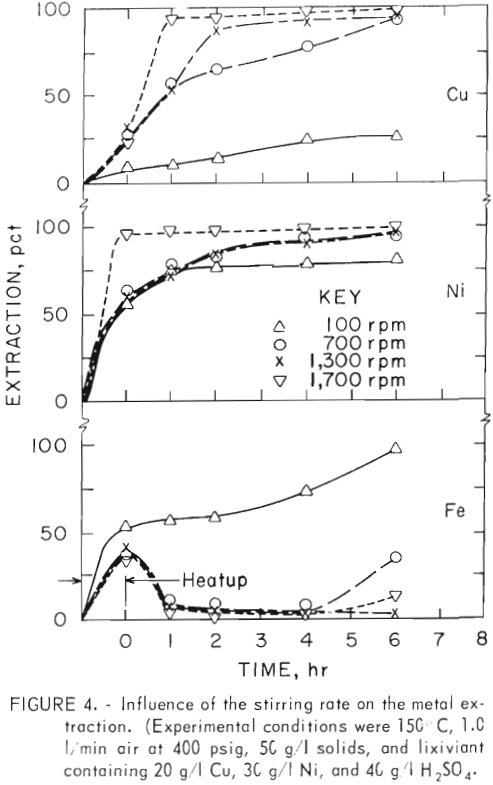 The extraction dependence on agitation speed and time is shown in figure 4. The iron extraction initially increased to over 30 pct regardless of the agitator speed but most of the dissolved iron was precipitated at agitator speed greater than 100 rpm. The higher iron rejection at the higher agitator speeds was attributed to the increased oxygen transfer and the oxidation of soluble ferrous ion to the less soluble ferric state. The copper extraction at 100 rpm was only 24 pct. Previous investigators have reported that the copper extraction is enhanced by the presence of ferric ion. Therefore, agitator speed of over 100 rpm are required to oxidize the ferrous ion to the ferric state and the latter appears to enhance the copper extraction.
The extraction dependence on agitation speed and time is shown in figure 4. The iron extraction initially increased to over 30 pct regardless of the agitator speed but most of the dissolved iron was precipitated at agitator speed greater than 100 rpm. The higher iron rejection at the higher agitator speeds was attributed to the increased oxygen transfer and the oxidation of soluble ferrous ion to the less soluble ferric state. The copper extraction at 100 rpm was only 24 pct. Previous investigators have reported that the copper extraction is enhanced by the presence of ferric ion. Therefore, agitator speed of over 100 rpm are required to oxidize the ferrous ion to the ferric state and the latter appears to enhance the copper extraction.
Effect of Airflow
The iron extraction-agitation data suggest that the oxygen transfer rate was important; therefore, the influence of airflow was evaluated at a fixed agitator speed of 700 rpm. At the 0.1-1/min airflow (test 13), insufficient oxygen was dissolved to oxidize the base metal sulfides to water-soluble sulfates. However, at 1.0 (test 10) and 2.5 (test 14) l/min, over 85 pct of the copper and nickel was extracted in 6 hours, and high-iron residues were obtained indicating that the ferrous ion was oxidized to ferric state.
Extraction Dependence on Particle Size
The influence of the matte particle size was determined at 1.0 l/min air. With matte II (greater than 98 pct minus 325 mesh), more than 95 pet of the copper and nickel was extracted at 400 psig and 180° C, but in order to obtain this extraction level with matte I (90 pct minus 325 mesh), higher temperatures (220° C) and pressures (550 psig) were required. The iron hydrolysis reaction was favored by high temperatures and pressures as indicated by the higher iron content of the residue in test 16 as compared with test 8.
Gas Composition Influence on Extraction
Matt II was used to determine the base metal extraction with the autoclave pressurized with oxygen rather than air. The results in tests 17-20 indicated that the nickel and copper extraction increased with increasing temperature and pressure. When the autoclave was pressurized to 100 psig with oxygen at 150° C, over 95 pct of the nickel and copper was extracted. However the iron rejection increased with increasing temperatures and pressures of 100 psig or greater (tests 18-20).
On comparing the oxygen and the air data, it is seen that the 88 plus nickel and copper extraction level was reached at 150° C when the autoclave was pressurized to 100 psig with oxygen or 400 psig with air. The steam saturation pressure at 150° C is 55 psig; therefore, higher oxygen partial pressure appeared to be required with air than with the pure oxygen. At the airflows used, 20 pct oxygen level was probably not maintained because some oxygen was consumed. Also, 100 pct oxygen was probably more effective in producing ferric ions which may have aided the oxidation of the copper sulfide to sulfate.
Leaching Behaviour of Matte Residue
Influence of Temperature & Pressure
Since the matte leach data indicated that the temperature and pressure were important variables, these parameters were investigated with the matte residue II. Tests 21-25 in table 3 indicated that the copper was the most difficult metal to extract. The 95-pct base metal extraction level was obtained when the autoclave was heated to 180° C and pressurized with air to 400 psig. These same general conditions were required with matte II (test 8).
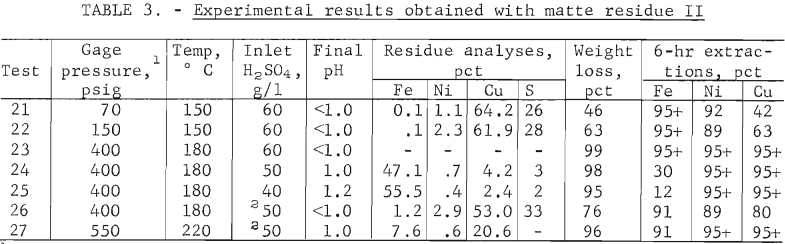
Effect of Lixiviant Composition
The influence of the nickel content in the lixiviant on the leaching characteristics of matte residue was investigated. The nickel concentration of the recycled spent electrolyte, shown in figure 1, depends on the percent nickel extracted in the first stage and the percent electrolyte bleed from the copper electrowinning circuit. Two nickel levels were investigated. Tests 5-25 were conducted with 30 g/l Ni, but in tests 26-27, the nickel content was increased to 70 g/l by adding nickel sulfate to the 30 g/l Ni, 20 g/l Cu, and 50 g/l H2SO4 electrolyte. When this high-nickel lixiviant was used, the nickel extraction dropped. The yellow residue that was formed in the autoclave was washed with cold water. The X-ray diffraction pattern of the solid indicated that nickel sulfate monohydrate was present. These results suggested that using a 70 g/l Ni lixiviant may have resulted in supersaturating the liquor at operating temperature. This possibility will be discussed further in the liquor studies section.
Liquor Studies
In a typical autoclave test with matte or matte residue and a lixiviant containing 30 g/l Ni and 20 g/l Cu, the resulting liquor at the 95-pct metal extraction level contained about 35 g/l Ni, 50 g/l Cu, and 2 g/l Fe. With this liquor, essentially no (15 pct) analysis difference was observed with liquid samples taken at operating temperature of 150° C or at ambient conditions. However, at 220° C (test 16), slightly lower nickel and copper liquor analyses were obtained just before the autoclave was shut down and after the liquid-solid separation step. A liquor nickel concentration difference was also noticed with the lixiviant containing 70 g/l Ni (tests 26-27).
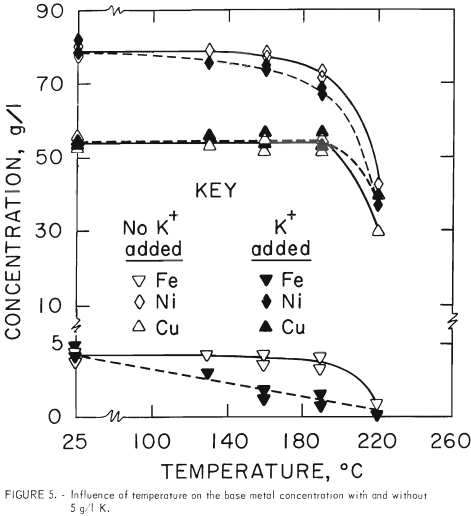
To determine the reason for the lower base metal liquor analyses at operating temperature, two nickel-rich liquors with and without potassium were investigated. Liquor I, without potassium and containing 78 g/l Ni, 55 g/l Cu, 4 g/l Fe, and 30 g/l H2SO4, was placed in the autoclave without matte or matte residue. The autoclave was pressurized with air to 550 psig, and the temperature was increased to various specified levels and held for 0.5 hour. It is evident from figure 5 (solid curves) that the base metal content did not drop at <130° C, but at 190° C, a slight decrease in the nickel concentration was observed. At 220° C, only about half of the nickel remained in solution. The copper and iron concentrations also dropped above 190° C.
The influence of potassium on the base metal solubility was also determined because some potassium would remain in solution after the potash-iron removal operation as shown in figure 1. Potassium sulfate (5 g/l K) was added to liquor I and the temperature-solubility tests were repeated. It is evident from the curves in figure 5 that the greatest drop in solubility occurred with iron. The iron content started to decrease at 130° and dropped to less than 1 g/l at 220° C. The residue from this test was washed with hot water, and the X-ray diffraction pattern of the solid indicated that potassium jarosite [KFe3(SO4)2(OH)6] was present. This material has been reported to be useful as a fertilizer.
Residue Data
The solids (remaining after 6 hours of autoclave leaching matte or matte residue) were classified into three color groups:
- A. Black residue.-These solids resulted when less than about 50 pct of the copper was extracted, and no visual change in the residue was noticed.
- B. Blue-black residue.-These solids were produced when over 90 pct the copper was extracted.
- C. Reddish-brown residues.-These solids had about the same extraction level as group B but the pH of the final liquor was equal to or greater than 1.
X-ray diffraction patterns of these residues indicated that the principal mineral in group A was bornite; in group B, covellite; and in group C, hematite. The blue-black and reddish-brown residues generally contained less than 0.5 pct Ni. The blue-black residues were principally copper sulfide, while the reddish-brown residues (usually contained less than 3 pet Cu) were mainly iron oxides.
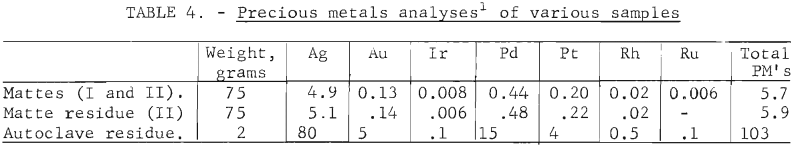
The total precious metals (PM) analysis of an autoclave residue, with greater than 95 pct of the base metal extracted at a final pH of less than 1, is given in table 4. The PM analysis of the autoclave residue is more than 15 times greater than that of the matte or matte residue, but weight reduction was over 30 times greater. No precious metals were detected in the liquor, which indicates that the precious metals were quantitively contained in the solids . An accurate material balance was difficult with the small quantity of sample available in these tests.
Summary
The metal extractions obtained with high-pressure (100 psig with oxygen at 150° C or 400 psig with air at 180° C) leach of matte residue or matte were about the same. The 95-pct extraction level was obtained at a matte grind of over 98 pet minus 325 mesh. However, at 90 pct minus 325-mesh grind, 220° C was required to obtain the 95-pct extraction level.
The 220° C temperature required with coarse matte resulted in a supersaturated solution at operating temperature when the autoclave was pressurized with air to 550 psig. The maximum metal content that remained in solution at this temperature was 40 g/l Ni, 30 g/l Cu, and 1 g/l Fe. This would mean that in order to extract all the copper from the matte, no copper could be tolerated in the lixiviant if the matte or matte residue were leached at 220° C and 50 g/l solids. Since spent copper electrolytes generally contain at least 20 g/l Cu, these results indicated that the matte must be ground finer than 90 pct minus 325 mesh.
The iron level remaining in the liquor after 6 hours of leaching was dependent on the pH and temperature-oxygen pressure. At a final pH of less than 1, essentially all of the iron was extracted. However, at pH values greater than 1, most of the iron was precipitated, especially when sufficient oxygen was transferred from the vapor to the liquid phase to oxidize the ferrous ions. At airflows equal or greater than 1 l/min and agitator speeds of 1,300 and 1,700 rpm, over 60 pct of the iron was oxidized to the insoluble ferric state when the final pH was greater than 1. The higher agitator speeds (1,300 and 1,700 rpm) also decreased the time required to extract 95 pet of the copper because only 2 hours were required as compared with 6 hours at 700 rpm.
The autoclave tests produced an enriched precious metals material. When over 95 pct of the base metals were extracted, the PM analysis of the undissolved solids was over 15 times greater than that of the matte or matte residue. The total PM analysis of the residue was more than 100 oz/ton.
The U.S. Department of the Interior, Bureau of Mines, is conducting laboratory-scale research to determine the leaching conditions required with sulfuric acid for extracting base metal from a matte with a copper-to-nickel ratio of 6 to 1. The matte (61.2 pet Cu, 9.7 pet Ni, 3.3 pet Fe, 21 pct S, and about 5 oz of precious metals per ton) was prepared from Duluth Complex (Minnesota) ore by smelting a bulk sulfide flotation concentrate. The matte was preleached at atmospheric pressure to selectively extract 20 pct of the nickel, and the undissolved solids were referred to as the matte residue. Autoclave leaching of the matte residue extracted over 95 pct of the nickel, copper, and iron. The remaining leach residue in the autoclave contained over 100 oz of precious metals per ton. The leaching conditions required were matte grind of greater than 98 pet minus 325 mesh, pulp concentration of 50 g/l, total pressure of 100 psig with oxygen at 150° C or 400 psig with air at 180° C, 50 g/l H2SO4, 700 rpm agitator speed, and 6 hours of leaching time. The leaching time was decreased to 2 hours by increasing the agitator speed to 1,300 rpm. Sulfuric acid concentration greater than 50 g/l did not appreciably enhance the base metal extraction, but with less acid, residues with higher iron concentrations were obtained. Supersaturation of the liquor was prevented at 150° C by using a lixiviant that contained 30 g/l Ni and 20 g/l Cu.
