Some epithermal veins, worked for gold in the upper, enriched zones, consist mainly of base-metal minerals below the enriched zone. The Smuggler vein at Telluride, Colorado, as shown by Lindgren, is an outstanding example. Its sulphides constitute 2 or 3 per cent of the vein and include principally pyrite, galena, zinc blende, and chalcopyrite accompanied by gold. Its gangue minerals are quartz, calcite, siderite, rhodochrosite, adularia, barite, and fluorite. The Sunnyside mine, at Eureka, Colorado, is also worthy of special mention, as its ore contains a greater percentage of base metals than that of the Smuggler vein and in recent years has been treated by selective flotation with the production of high-grade zinc concentrates, high-grade lead-copper concentrates which contain most of the recovered gold and silver, and zinc-lead-copper-silver-gold middlings. The Shenandoah-Dives vein southeast of Silverton, which also formerly was worked at higher levels for gold and silver, is now worked mainly for lead-copper-zinc sulphide ore containing gold and silver. Lindgren notes that the mines of the Silverton district contain more base metals than the majority of epithermal veins, and on the whole resemble the deeper seated veins; also, that their variety of gangue minerals is typical of the epithermal zone except that their quartz has a coarser granular texture than is common in veins deposited nearer the surface. Similar examples, but less important commercially, could be cited from the Colorado Front Range, from western Montana, and from the Cascade region of Western Oregon, and would lend further support to the suggestion that the mineral composition of a vein depends largely on local conditions. An impervious barrier might retard the movement of a solution until all its contents were deposited in a definite sequence within a single ore shoot; or only the earlier gangue and sulphide minerals might be deposited at one place while the solution moved on to deposit the later gangue and precious- metal minerals at another place; or the minerals of the earlier stage might become reopened and allow those of the precious-metal stage to fill fractures in them or to replace them. The relations of epithermal to mesothermal deposits will doubtless become more clearly demonstrated as more attention is given to the sequence of mineral deposition and to the idea that deposition may take place under several successive changes in temperature and other physical conditions even within a single ore shoot.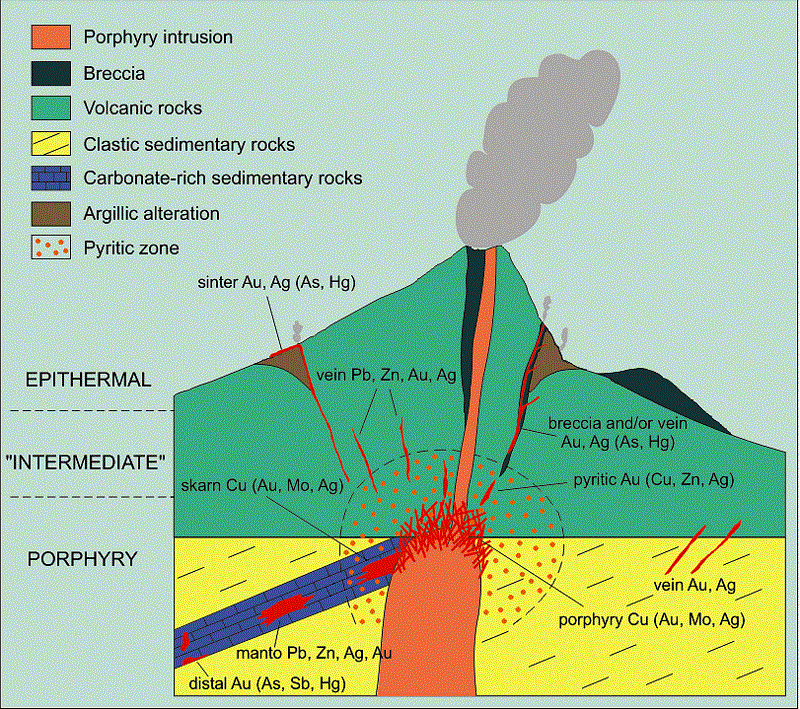
Where limestones and dolomites are the predominating country rocks, epithermal deposits are likely to have very different features from those in volcanic rocks. Such differences, as already suggested, may be attributed in part to original differences in composition of ore-forming solutions and in part to reactions between the solutions and the country rocks. It may be too great a stretch of the imagination to assume that large deposits of zinc and lead sulphides in limestones could have been deposited by the same original solutions that deposited the gold-quartz- adularia veins in volcanic rocks; furthermore, since the lead-zinc deposits are commonly associated with plutonic rocks of intermediate composition (monzonite and diorite) whereas the gold-quartz-adularia deposits are commonly associated with volcanic rocks that show a considerable range in composition, there is no convincing reason for believing that they had to be derived from originally identical solutions. Reactions between solutions and country rocks, however, tend to emphasize differences between the two kinds of deposits. Siliceous rocks, including volcanic flows, are only slowly attacked by these solutions, whereas carbonate rocks are rapidly attacked; deposits in siliceous rocks, therefore, may be distributed over a relatively long distance and their lead and zinc minerals may be correspondingly scattered in shoots too small to be profitably mined, whereas deposits in carbonate rocks are likely to be concentrated within a short distance and their lead and zinc minerals segregated into large shoots. Obviously, local structural conditions may modify this purely chemical generalization. For this reason places may be cited where large zinc-lead deposits occur in siliceous rocks, though probably not in the epithermal zone, and other places where they are small and scattered in carbonate rocks; but, if each locality is studied with special reference to the reacting value of wall rock this generalization will find support.
The reacting value of carbonate rocks may be so great as to extract the bulk of the metallic contents from a solution of magmatic origin before the epithermal zone is reached, and the modified solution that reaches the epithermal zone may contain little besides the constituents of dissolved carbonates, which are ultimately deposited successively as dolomite and calcite in any kind of country rock. Any lead, zinc, or other metals that succeed in reaching the epithermal zone are deposited as sulphides, but few if any deposits that can be referred to such an origin contain noteworthy amounts of gold and many are also low in silver. If these minerals were present in greater quantity in the original solutions, they were evidently precipitated in some deeper zone, probably by reaction with certain sulphides deposited during an early stage. This kind of reaction adds one more complication to the problem of classifying deposits into distinct zones.
Another complication that remains to be considered is expressed in the great range in depth throughout which lead-zinc deposits have been formed in different limestone regions, despite the fact that their mineral compositions indicate deposition in the epithermal zone. Estimates of the amount of erosion that has taken place since ore deposition show that some were indeed deposited at shallow depths but that others may have formed as much as 10,000 ft. below the surface, although a long way from their deep-seated source.
This variety of epithermal deposit, or better “telethermal” deposit, since it was formed far from its thermal source, has not been especially mentioned in Lindgren’s chapter on epithermal deposits, and it has probably been regarded by many as belonging either to the mesothermal zone, with the great majority of zinc-lead deposits, or, if its origin were not clearly related to igneous activity, to the class of deposits formed by circulating atmospheric waters at moderate or slight depth.
