Bacterial extraction of gold from sulfide bearing ores has been studied by many investigators. If gold occurs finely disseminated within the sulfide ore matrix such as in pyrite, arsenopyrite and pyrrhotite, the leachant can not penetrate into the inside of solid ore in order to leach the enclosed submicron size gold particles. For their liberation, the sulfide bearing ore is subjected to preoxidation by bacterial leaching. During the bacterial leaching, pyrite is oxidized to yield ferric sulfate and sulfuric acid:

The pyrite oxidation increases the porosity of solid substrate and the follow-up gold extraction by NaCN or thiourea solutions. The gold extraction from gold-bearing pyrite is dependent on the yield of bacterial pyrite preoxidation. However, the metabolic energy of microorganisms in oxidizing pyrite is influenced by the inhomogeneity of mineral surface, dissolved oxygen concentration, the iron redox cycle and varying concentration of ferrous, ferric, heavy metal (impurities) and gold ions, which are released dining the bacterial leaching. The metabolic activity is furthermore complicated by the fact that pyrite is a semiconductor and shows non-stoichiometry between its iron and sulfur contents (as well as impurities). It may naturally occur in n- or p-type. Therefore, the conduction in pyrite takes place when electrons are exited into eg orbital. Pure pyrite is known to resist to chemical dissolution because its valence band is of non- bonding character, and the holes do not contribute to bond breaking. The present study aims to investigate the intermediate reactions involved in the bacterial leaching of gold-bearing pyrite using an adapted culture of Thiobacillus ferrooxidans and electrochemical techniques, such as cyclic voltammetry, chronopotentiometry and chronoamperometry. Furthermore. the extraction of gold will be achieved from the leach residue of bacterial leaching by thiourea solutions.
Bioleacling Experiments
This series of experiments were carried out in 250 cm³ Erlenmeyer flasks containing 5g high-grade ore or 32 g low-grade ore and 100 cm³ of nutrient medium with 5 cm³ bacterial inoculum of T. ferrooxidans. After pyrite biooxidation the leach residue was leached by thiourea solutions to extract gold.
Mechanism of Gold — Bearing Pyrite Leaching
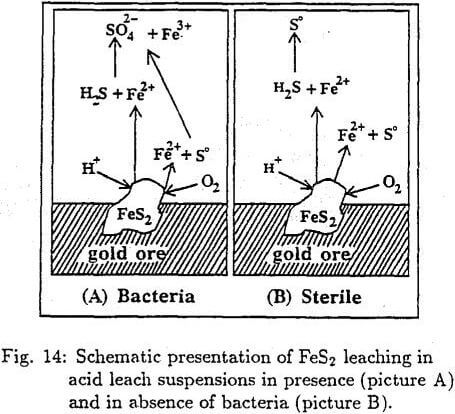
The mechanism of pyrite oxidation can schematically be presented as shown in Fig. 14. In the absence of bacteria the final product of pyrite oxidation is ferrous ion and elemental sulfur and in the presence of bacteria ferric and sulfate ions.
The principal advantage of the presence of bacteria is that it results in continuous production of Fe³+ ion, which is essential for the gold extraction. Therefore, to shed more light on the production of Fe³+ ion, the pyrite oxidation mechanism must be explained. Fig. 15 illustrates the schematic energy density states. The relative position of Ef of redox leach system to the decomposition energy level Ed of pyrite provides information about the chemical stability of pyrite . Therefore n-type pyrite is easily oxidizable by the microorganisms while the p-type pyrite is not. Note that in the p-type pyrite the Ef is located close to the energy level of the valence zone. Therefore the p-type is very stable and difficult to be oxidized.
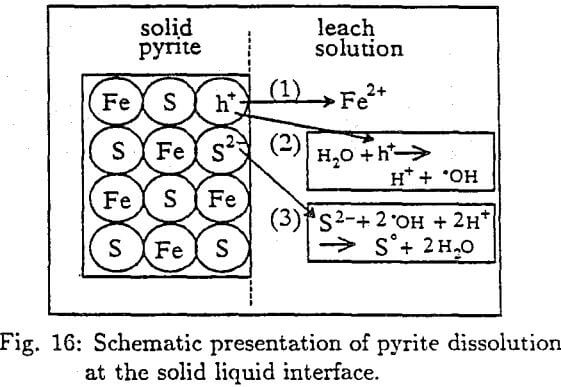
When Fe²+ ion leaves the solid pyrite crystal an excess of negative charge, S²-, is left behind. This will establish a potential difference between the solid pyrite and the nutrient solution that will tend to prevent the movement of Fe²+ ion into the bulk of the solution. The S²- is not immediately free to move out of the crystal since its energy level is in the filled valence band. However, the excess of negative charge of S²- can be assisted to be neutralized by the hole (h+), that were left behind when Fe²+ moved into solution. The hole reacts with water to yield hydroxyl radical :
H2O + h+ →H+ + OH……………………………………………………………..(24)
The OH radical can now react with S²- ion by eliminating the excess negative charge and forming S° that can further be oxidized to H2SO4.
S²- + 2 · OH + 2H+ → S° + 2H2O………………………………………………………(25)
As a result, the excess negative charge is eliminated and the Fe2+ ion is free to move into the bulk of the leach solution.
