The fact that gold will dissolve in sodium hyposulphite solutions is known from the lixiviation of silver and gold ores. The gold will dissolve only in small quantities. According to researchers, if gold leaf is treated for a time with sodium hyposulphite solutions of varying strengths,’ solution takes place in every instance, the deviation in quantity being very slight. ‘
In order to form hyposulphite double salts, the gold must become oxidised, the oxygen being derived from the air absorbed by the solution. Consequently, it is the quantity of free oxygen in the solution by, which the reaction is determined, as for one equivalent of gold dissolved one equivalent of caustic soda is set free.
The quantity dissolved depends upon many circumstances; but is, principally, a matter of time. It appears that a solution of sodium hyposulphite prepared by the addition of acetates and perchloride or bromide of iron acquires the property of dissolving gold and silver more rapidly than under usual conditions, as follows.
If perchloride or bromide of iron is added, drop by drop, to a solution of sodium hyposulphite, every drop so added will produce a black violet colour in the solution, which on stirring will disappear. This solution possesses the property of dissolving gold and, silver. Being a very unstable compound it will’ undergo a rapid decomposition and separate sulphur from its solution. By the addition of more perchloride or bromide of iron, a point is reached where the solution takes the colour of perchloride of iron, and the property of dissolving gold and silver is lost.
If to a solution of sodium hyposulphite, a solution of acetate of iron peroxide or any other acetate, such as acetate of soda or lime, is added, with perchloride or bromide of iron, the solution will turn dark red, the known reaction for acetic acid, and will become darker in colour by further additions of perchloride of iron. This solution, so prepared, will easily dissolve gold and silver, and in all probability the known reaction will take place. Hyposulphite double salts are formed; the precipitation of the gold from this solution by nitric acid, zinc, or electrolysis, but not by protosulphite of iron or sulphurous acid, shows this. The chemical explanation may be as follows:
The oxidisation of a portion of the hyposulphite solution forms a protooxide of gold, which combines with a portion of the undecomposed sodium hyposulphite to form a double salt.
The solution will gelatinise, according to concentration and composition. This gelatinisation can be prevented by the addition of a free acid, such as acetic acid.
The addition of an oxidising agent like sodium peroxide or bichromate of potash will increase the solution of the gold considerably; but a gradual separation of sulphur from the solution will take place.
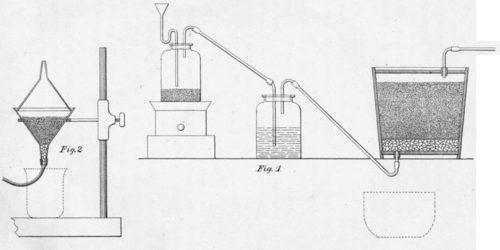
These solutions have very little solving action upon the natural sulphide contained in gold-bearing ore, and should be suitable solutions for the treatment of gold or silver ore, as they have a greater solving power than cyanide of potash, are not poisonous, and can be obtained in very large quantities at a very low price. For instance, a solution of 1 or 2%, of sodium hyposulphite, 3/4%, of perchloride of iron and 1 or 2%, of soda or lime acetate, diluted up to 10 times its volume, is suitable for the lixiviation of the various gold ores.
With the same volume and the same exposed surface of gold, the above solution will dissolve from within 6 to 12 hours, 15 or 24 times as much gold as a 1 or 2%, cyanide of potash solution in the same time.
The application of the process by the above solution is nearly the same as that by cyanide of potash. The precipitation of the gold can be affected by zinc shavings or electrolysis or by saltpetre with sulphuric acid.
Advantages of roasting ores for cyanidation
The advantages gained by roasting the ores previous to cyaniding are as follows:
The consumption of cyanide is less, percolation easier, and less caustic soda or lime is required for neutralization. To test whether the ores are properly roasted,’dissolve 100 to 250 grams of the roasted product in a glass beaker, stir well, filter, and add to the solution, cyanide of potash of the same strength of solution as used in the treatment on a large scale. If by the addition of the cyanide of potash solution, no clouds are produced in the solution the ore is well roasted. If the solution is coloured brown, the consumption of cyanide will be large, and if the solution gives a blue or blue-green precipitate, the ore is imperfectly roasted.

