The Bureau of Mines has conducted extensive research to improve the extractive technology of gold and develop processes which may be applicable to refractory ores. Some metallurgists believe that the recovery of metallic values from ores and concentrates by vapor-transport techniques has great potential in the field of extractive metallurgy. The purpose of this phase of the Bureau’s research was to study the volatilization of gold.
Chloride volatilization of gold has been known for about 90 years. The process is used to extract gold, silver, and other nonferrous metals from pyrite cinders, but it has never been successfully applied on a plant scale to gold ores. Early metallurgical operations involved salt-roasting refractory silver-gold ores with sodium chloride (NaCl) or calcium chloride (CaCl2) to form soluble chlorides, which could then be leached. A major disadvantage of this process was that considerable gold was lost during the roasting of some ores. In addition, the results obtained in these operations were unexplainably inconsistent, and the reaction mechanisms were never well defined. Christy was the first to document the gold losses sustained by several operating metallurgists. For example, he recorded Aaron’s loss of gold in salt-roasting a pyrite ore, and Stetefeldt’s losses of 43 to 93 percent of the total gold in roasting a pyrite-chalcopyrite (CuFeS2) ore. He similarly recorded that Butters volatilized 70 to 85 percent of the gold from a pyrite-bearing ore. Christy then investigated the effect of chlorine and temperature on the volatilization of gold and found a region of high volatility at 200° to 300° C, low volatility near 600° C, and increasing volatility above 600° C. The loss of gold was attributed to the formation of gold chloride.
Around 1900, a process was introduced that utilized the volatility of gold chloride instead of trying to suppress it. In the Pohle-Croasdale process, ore was roasted with sodium chloride at 750° to 1,050° C, and the volatile chlorides were separated from the gangue. Difficulties were encountered, however, in condensing the chlorides, and no successful means for doing so were found. Other metallurgists, studying salt roasting at lower temperatures, noted that their results varied from ore to ore and sometimes varied within an ore. In certain ores the gold volatilized below 600° C, whereas in others it did not. Hence, to insure consistent gold volatilization, it was necessary to go to high temperatures as in the Pohle-Croasdale method.
Mather, in 1903, claimed it was possible to volatilize gold from ores at temperatures well below 700° C. He claimed to have accomplished the sublimation of all values in a copper-gold-silver ore. as compound chlorides at temperatures lower than 400° C. He also stated that under certain unexplainable conditions, a partial sublimation of some metal chlorides occurred at a temperature lower than the boiling point of water; however, this claim has never been verified. He theorized that metals volatilize as simple chlorides at high temperatures, but as compound metal chlorides at low temperatures.
In 1923, a group of investigators from the Bureau of Mines at Salt Lake City and the University of Utah studied the Pohle-Croasdale process. These investigators roasted a number of ores at 900° to 1,000° C with NaCl and applied the method on a bench and pilot plant scale. The Cottrell precipitator was used to effectively collect the volatile chlorides.
Biltz and coworkers studied the physical chemistry of gold chlorides in 1928 by passing chlorine gas over finely divided gold at various temperatures. They showed that gold chloride has a region of high volatility at 254° C, low volatility from 400° to 600° C, and increasing volatility between 600° C and the melting point of gold.
Further application of the chloride volatilization process to treat gold, silver, copper, and lead ores was made on a bench scale by the Bureau of Mines.
In the present research, the volatilization of gold as a chloride was investigated at low temperatures, in the range where results obtained by previous investigators had been erratic and unreproducible. Preliminary investigations on the volatilization of gold from ores showed that the ores were too complex to permit effective control of all parameters. Therefore, major emphasis was directed toward the study of a gold-silica system, which yielded consistent results and permitted a more precise definition of the mechanism of low-temperature gold volatilization. The discovery of an iron-gold chloride species that was volatile at low temperatures explains why the early metallurgists could not obtain reproducible results with various ores under identical treatment conditions.
Experimental Procedures
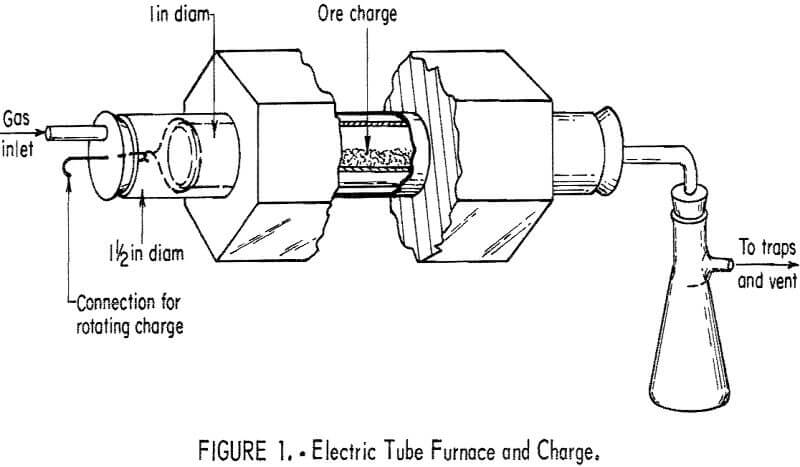
The chloride volatilization tests were conducted in a horizontal electric tube furnace containing a quartz tube, as shown in figure 1.
The gold-bearing charge was placed in a 1-inch-diameter quartz tube, which was inserted into the 1-½-inch-diameter tube of the preheated furnace. After the system was made airtight, the flow of chlorine was initiated. The charge was roasted 1 hour and rabbled every 15 minutes by rotating the inner quartz tube with attached wires that extended out through the rubber stopper. At the end of a run, the tube was withdrawn and allowed to air-cool before the charge was removed. A series of three traps was placed at the outlet of the furnace to condense fumes before they entered the exhaust system.
The initial tests were conducted on an oxide ore, ground to 100 mesh, which contained 0.40 oz gold/ton. The gold occurred as submicron-size particles in a porous gangue matrix.
The subsequent reaction studies were conducted on a pure gold-silica mixture in the presence of controlled amounts of additives. For these investigations, a master gold-silica mix containing 40 oz gold/ton was prepared in the following manner: Silica flour was first purified by digesting with aqua regia and then washed with distilled water until free of acid. An aqueous solution of gold chloride was added to a slurry of the purified silica flour,
and the gold was precipitated by adding oxalic acid solution, heating, and stirring. The resulting slurry was filtered, washed, and dried. Batches containing ~0.5 oz gold/ton were then prepared for use in the experiments by adding 10 grams of the master mix to 1 kg purified silica flour. The batch was tumbled for 24 hours; four assays made on each batch showed that this provided uniform mixing. The resulting mix was analogous to an ore containing finely disseminated gold in a porous siliceous gangue.
Minerals tested as sources of gold-complexing components were clean specimens ground to minus 100 mesh, including pyrite, galena (PbS), chalcocite (Cu2S), chalcopyrite, bornite (FeS·2Cu2S·CuS), covellite (CuS), wurtzite (ZnS), pyrrhotite (FeS), and millerite (NiS). Chemicals used as promoters were ferrous sulfide (FeS), sulfur, arsenic sulfide (As2S3), sodium sulfide (Na2S), ferric oxide (Fe2O3), and ferric chloride hexahydrate (FeCl3·6H2O). The materials were of reagent-grade purity and were ground to minus 100 mesh. Other chemical additives included impure crystalline aluminum chloride (AlCl3) and the sulfides of aluminum (Al2S3), gallium (Ga2S3), and indium (In2S3), which were added as lumps. Metal additives included chemically pure iron and aluminum powders, tin in the form of small shot, and electrorefined titanium metal crystals.
Reactions between the additives and the gold-bearing charge in the quartz tube were obtained by two techniques, which gave equally good results. In one method, a 20-gram charge, containing 0.2 to 0.3 mg gold, was mixed with the desired weight of additive (usually 0.25 gram) before being put in the quartz tube. In the other method, the additive was placed 3 inches in front of a 10-gram charge so that the stream of chlorine passing over the additive generated the desired vapor, which then passed over the charge. This was especially useful for additive materials that could not be finely ground.
Gold volatilization and transport were calculated by the difference in gold content of the starting material and the calcine as determined by fire assaying. Metallurgical balances were obtained in certain cases involving large amounts of gold by rinsing the traps at the furnace outlet and determining the gold content of the solution used to dissolve the condensed fume.
Results and Discussion
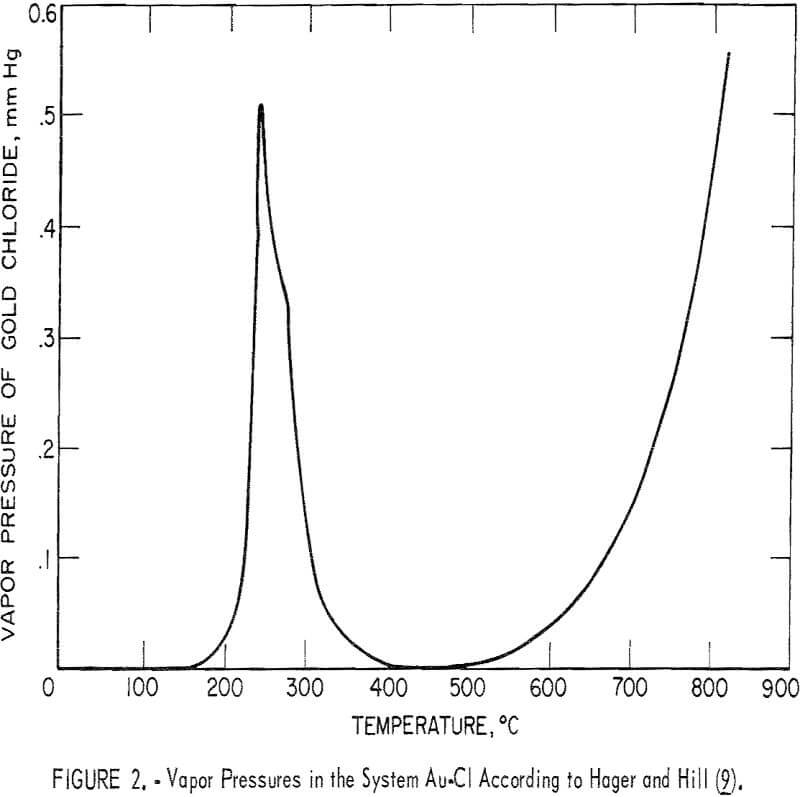
Figure 2 is a graph showing the vapor pressure of gold chloride versus temperature. The region of gold volatility at 250° C is very evident; this is due to the formation of the volatile gold chloride, Au2Cl6(g). As the stability of Au2Cl6(g) decreases with increasing temperature, the vapor pressure of Au2Cl6(g) becomes vanishingly small. There is an increase in vapor pressure, starting around 600° C, presumably because of a gold monochloride species.
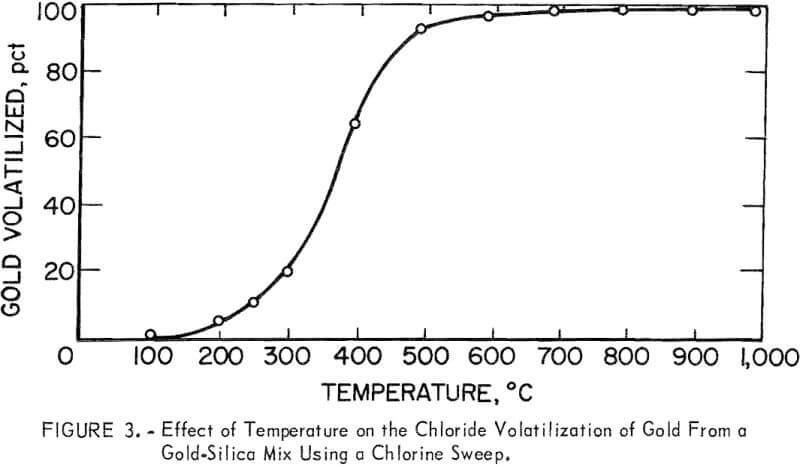
When the oxide gold ore sample was chlorinated, there was no gold volatilization at 250° C and no appreciable volatilization below 600° C. Because of the large number of constituents in the ore and the variety of reactions that can occur, further work was done using the synthetic mixture, which contained only gold and silica, to simplify the study of the mechanism by which gold is volatilized.
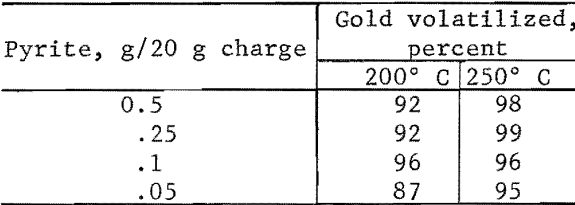
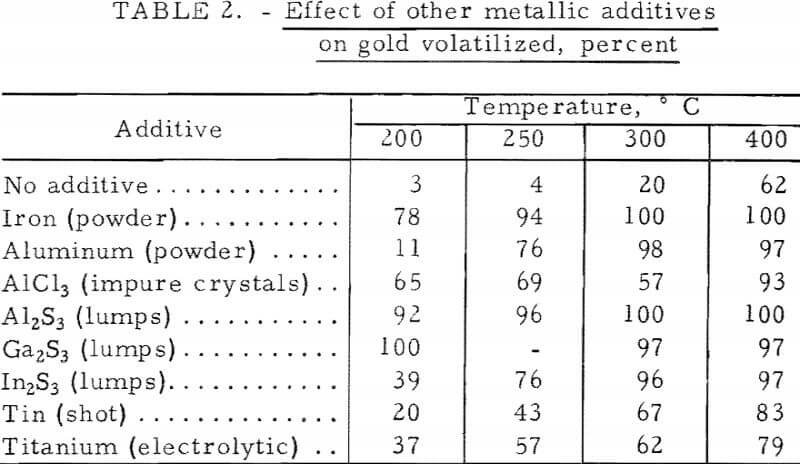
The results of chlorinating the gold-silica mixture are shown in figure 3 as the percent of gold in a charge volatilized in a 1-hour run. Although it would be expected that the most gold would volatilize at temperatures where the chloride has the highest vapor pressure, there was practically no gold volatilization near 250° C. Gold was volatilized, however, between 400° and 600° C, which is an area of nonvolatility according to figure 2. This would appear to be an anomaly; however, the results obtained for the gold-chlorine system cannot be applied to the gold-silica-chlorine system, especially where the gold concentration is very low as in low-grade ores. The different behavior of the two systems explains why gold has not been volatilized from ores via chlorination at 250° C, although it is well known that the metal chloride is volatile at this temperature. The basic chlorination behavior of the gold-silica mixture was established by repeated runs, which also showed that pure Cl2 and a CI2-plus-air mixture gave the same results. In subsequent experiments, the effect of additives was studied, and the results attained with the first group of minerals and chemicals are given in table 1. Of particular interest were the iron compounds, which greatly lowered the temperature at which gold could be volatilized. Elemental sulfur, ferric oxide, and hydrated ferric chloride did not promote volatilization when used separately, but when sulfur and an iron compound were combined, anhydrous ferric chloride (Fe3Cl6(g)) was produced and volatilization was enhanced. The degree of gold volatilization was not markedly dependent on the amount of pyrite used, as shown by the amount of gold volatilized under the following conditions:
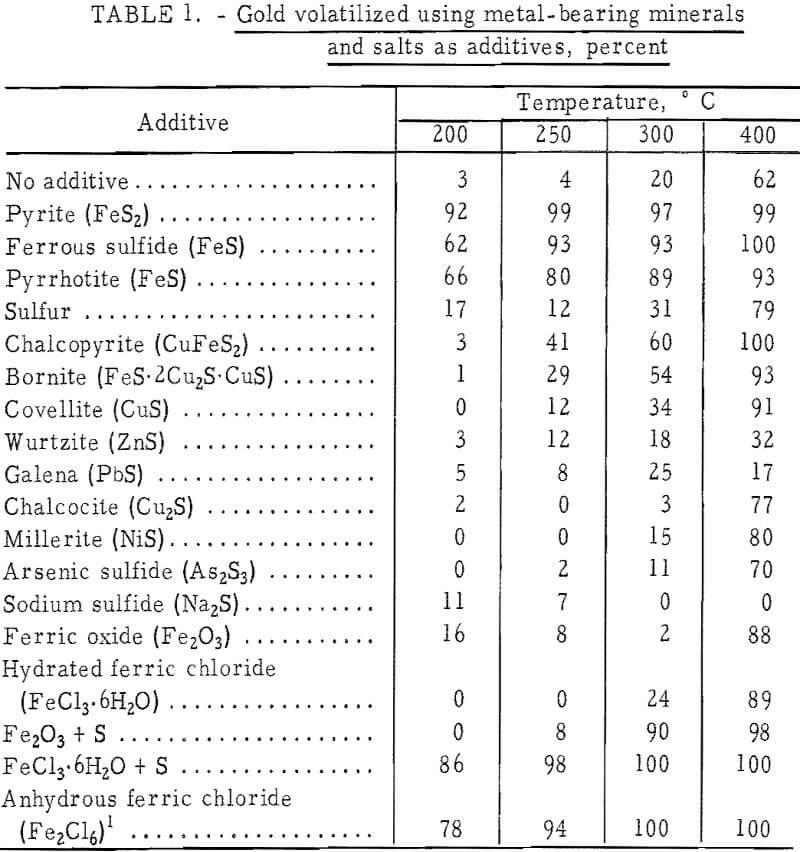
To determine what elements were present in the condensate, a large amount of material was volatilized and collected. A charge consisting of 0.5 gram of minus 325-mesh gold metal powder and 1.0 gram of pyrite volatilized at 250° C gave a condensate of very hygroscopic, dark red crystals, which a qualitative X-ray fluorescence scan showed to contain gold, iron, and chlorine. The sulfur content was 0.002 percent. This suggested that a mixed iron-gold chloride complex was causing the volatilization of gold at the low temperatures.
Substitution of pure iron powder for iron sulfides was equally effective for promoting gold volatilization in this temperature range (table 1).
As part of this project this conclusion was confirmed by Hager and Hill in their mass spectrometric study of the vapor transport reactions involving gold. A sample of gold and iron heated in a Cl2 atmosphere resulted in the detection of a number of iron-gold-chlorine ionic species, which were identified as originating from the parent species FeAuCl6(g).
The similarity between the vapor-phase species of iron and gold chlorides suggests that the iron-gold chloride may be formed by simple substitution of a gold for an iron atom. As shown in figure 4, both chlorides are dimers in the vapor phase with chlorine bridges between the metal atoms. In Fe2Cl6(g) each iron bonds four chlorine atoms by a tetrahedral sd³ configuration, with two tetrahedra sharing a common edge in the dimer. In
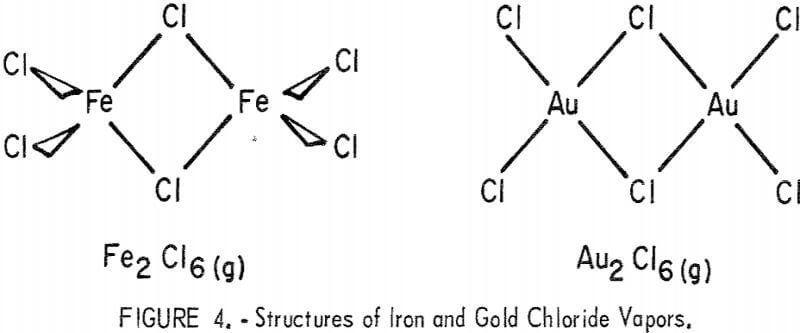
Au2Cl6, each gold bonds four chlorine atoms in a square planar dsp² configuration to form a planar dimer molecule.
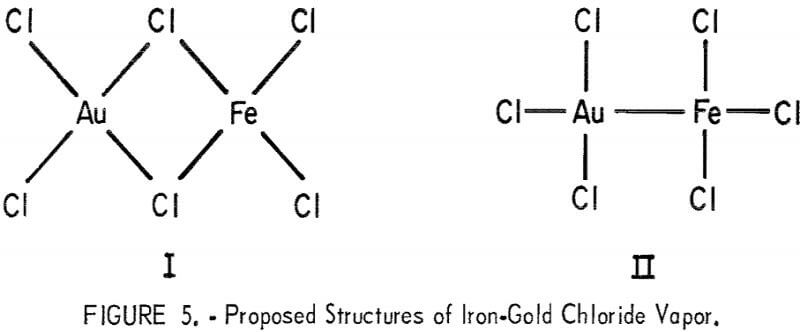
It is proposed that iron-gold chloride has structure I or II shown in figure 5. Structure I would be expected from known characteristics of gaseous iron and gold chlorides. However, the fragment AuFe+ was detected in the mass spectrometer; this would presumably come from a structure such as II. It is possible that the molecule is predominantly chlorine bridged with some intermetallic bonding character.
Aluminum also forms a dimeric trichloride, which is volatile and very similar to Fe2Cl6(g) in structure and may be capable of forming a mixed chloride with gold. Table 2 gives the results with a number of additives of the iron and aluminum families. All are effective promoters of gold volatilization at low temperatures. Indium sulfide is mainly monomeric in the vapor state rather than dimeric as are the aluminum and gallium sulfides; perhaps for this reason, it is a less effective promoter. Tin and titanium form extremely volatile tetrachlorides rather than trichlorides, but are found to aid gold volatilization.
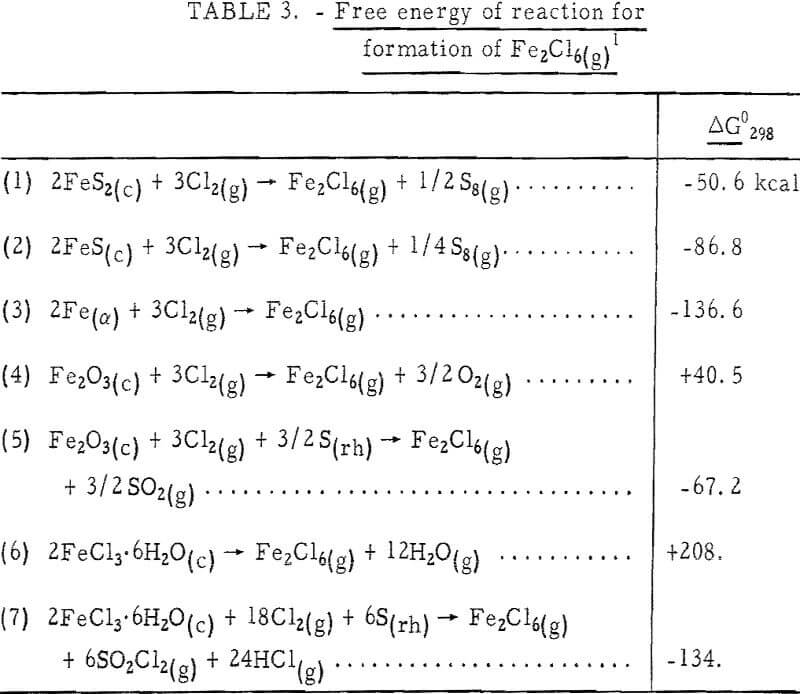
An examination of thermodynamic values offers an explanation of why certain ferric salts do not promote volatilization except in the presence of sulfur. If we assume formation of Fe2Cl6(g) is a necessary prerequisite to aid gold volatilization, calculation of the free energy change on formation should indicate which reactions will take place if the reaction rate is favorable. Table 3 gives the reactions and free energy changes involved in the formation of ferric chloride vapor from various materials. The ΔG° values are negative for iron and the sulfides and go from positive to negative on the addition of sulfur to Fe2O3 and FeCl3·6H2O to allow formation of SO2 and sulfuryl chloride (SO2Cl2). Although FeCl3·6H2O might be expected to form Fe2Cl6 vapor by simply heating, this is not the case. Wells states that in ferric chloride hexahydrate the central iron atom is bound to the molecules of water rather than to chlorine, and anhydrous ferric chloride cannot be recovered by heating.
Gold vapor transport remained constant over a wide temperature range with FeS2 as a promoter; 97 to 100 percent of the gold was volatilized at temperatures from 300° to 1,000° C.
Conclusions
Although metallic gold reacts with chlorine at temperatures near 250° C to form a volatile chloride, Au2Cl6(g), the amount of gold volatilized at this temperature from a gold-silica mixture with a chlorine sweep was small. It was established that iron and aluminum compounds enhance gold vapor transport in this temperature region by formation of a volatile complex metal chloride for which a structure is proposed. Tin and titanium also aid gold volatilization, although not as effectively as iron and aluminum, but whether they form similar complexes has not been determined.
In the recent literature, there are reports of vapor-phase complex chlorides for divalent transition metal, alkaline earth, lanthanide, and actinide chlorides. However, these have different stoichiometric compositions than the covalent complex chlorides described here.
The formation of a volatile iron-gold chloride special offers an explanation for the observations made by early metallurgists who applied the salt-roasting process to precious metal ores; namely, that gold was lost by volatilization from some ores, but not from others. Most of the recorded losses were from pyritic ores; pyrite reacts to form the volatile complex chloride at very low temperatures. As iron is present in virtually every ore, it may well have reacted with gold over the entire temperature range. The minimum temperature at which the reaction would proceed with any given iron compound would be limited by the reaction of the iron mineral with NaCl to produce Fe2Cl6(g). On the other hand, compounds such as Na2S and ZnS were shown to suppress low-temperature gold volatilization (carbon also effectively suppresses volatilization) , and the presence of such materials would reduce gold losses at low temperatures.
The discovery of this type of volatile species offers interesting possibilities in extractive metallurgy for devising new methods or modifying existing processes for the recovery of metals from their ores through the formation of complex vapor transport species.
