Table of Contents
The purpose of the Bureau research described in this report was to devise a procedure for producing TiCl4 from titaniferous raw materials, especially those that are high in CaO content. This research supports the Bureau’s goal of maximizing the recovery of minerals and metals from domestic resources in order to assure an adequate supply of minerals to meet national economic and strategic needs.
Although titanium-bearing materials are found throughout the world, the current demand for titanium ores is primarily satisfied by only two minerals, These are rutile, which is 95 pct TiO2, and high-grade ilmenite (FeO·TiO2), which is more than 55 pct TiO2. World reserves of rutile are limited. In the United States, reserves of lower grade ilmenite and other titaniferous materials are abundant, but the country is nonetheless dependent on foreign sources for about 65 pct of the titaniferous raw material used in manufacturing TiO2, welding rod coatings, and titanium metal.
In 1976, the domestic industry produced 713,000 short tons of TiO2. However, certain waste disposal problems are associated with processing ilmenite or titanium smelter slag in the production of TiO2. Titanium dioxide for the pigment, paper, and plastic markets is manufactured by domestic producers using either a sulfate or a chloride process. When the sulfate process is used, ilmenite or titanium slag is first dissolved in H2SO4. This is followed by separation of the iron sulfate and subsequent hydrolysis of titanyl sulfate to hydrated TiO2 , which is calcined to TiO2. The iron sulfate and acid wastes are disposed of in the oceans and other waterways.
The newer chloride process is used for approximately 65 pct of domestic TiO2 production and is environmentally preferable to the sulfate process because less waste material is generated. To produce TiO2 by the chloride process, rutile or ilmenite feedstock is chlorinated to produce TiCl4, which is then purified and oxidized to pigmental TiO2 or reduced to sponge metal by reaction with sodium or magnesium. Chlorination of ilmenite generates iron chloride and other metal chloride wastes that cause a potential pollution problem when disposed of in waterways or injected into deep wells.
For each pound of TiO2 produced from ilmenite, chloride processing generates 1.2 pounds of waste material, compared with 4 pounds of waste generated from sulfate processing. Sulfate processing of titanium slag made from ilmenite generates about 2.6 pounds of waste per pound of TiO2 product. Chlorination of natural rutile, however, generates only 0.5 pound of waste since the total impurity content of the feedstock is usually less than 5 pct.
Quebec Iron and Titanium Corp. (QIT) in Sorel, Quebec, Canada, employs the only commercial process for upgrading ilmenite to a titanium-rich slag. This slag is used as feedstock in sulfate-process plants that produce TiO2 pigments. The slag process used by QIT consists of reduction smelting ilmenite-coal mixtures in an electric arc furnace to separate about 85 pct of the iron as byproduct pig iron and to produce titanium slag containing about 72 wt-pct TiO2, 13 wt-pct FeO, and other oxide impurities. In 1975, QIT produced about 900,000 tons of titanium slag. QIT has recently built a commercial smelter at Richards Bay in the Republic of South Africa to produce titanium slag from a high-grade ilmenite sand concentrate.
Increased consumption of rutile in chloride-process TiO2 production has driven natural rutile prices up to more than $500 per ton. This has stimulated worldwide interest in upgrading ilmenite to a high-TiO2-content feedstock for chlorination. As a result, four new processes have been developed by industry on a semicommercial basis to produce a synthetic rutile product containing 90 to 95 wt-pct TiO2 from high-grade ilmenite sand concentrates containing 55 to 65 wt-pct TiO2. In the three processes developed outside the United States, the iron oxides contained in ilmenite are selectively reduced to the metallic state and subsequently separated from the TiO2 by chemical extraction or by physical separation. In Australia, Western Titanium N. L. annually produces 43,000 tons of synthetic rutile containing 93 to 94 pct TiO2. In India, Dhrangadhra Chemical Works, Ltd., produces 25,000 tons per year of a product that is 90 pct TiO2. In Japan, Ishihara-Sangyo Koisha, Ltd., operates a 30,000-ton-per-year synthetic rutile plant at Yokkaichi. A domestic pigment producer operated a synthetic rutile plant near Mobile, Ala., but the plant recently ceased production because of the availability of lower priced imported chlorination feedstock. The Alabama plant, which had a capacity of 110,000 tons per year of synthetic rutile, was based on HCl leaching of ilmenite.
The Bureau has devised a new technique for producing and chlorinating TiC feedstock from domestic titanium-bearing raw materials. A system was devised to convert either perovskite or ilmenite ore concentrates to TiC, which was chlorinated to produce TiCl4. This report discusses initial studies on carbiding tests performed on titanium slag made from ilmenite using larger scale equipment. The response of purified TiC to chlorination in bench-scale tests was favorable, and the results of these tests are included in this report. (This research employed techniques that result in a minimum amount of waste and environmental degradation.)
Experimental Procedures and Results
Two samples of domestic titaniferous materials, a perovskite concentrate and an ilmenite concentrate, were treated. The perovskite was a calcium titanate (CaTiO3) derived from a massive rock deposit located in the Cebolla Creek region near Powderhorn, Colo. The perovskite concentrate was prepared by a commercial laboratory on an experimental basis, but only a 200-pound sample was available. To produce the concentrate, the perovskite ore was beneficiated using low-intensity wet-magnetic separation of the magnetite and gravity concentration (tabling) of the perovskite in the nonmagnetic tailing. Mineralogically, the concentrate was a cerium-bearing perovskite associated with minor amounts of accessory minerals, including pyroxene, magnetite, and quartz. Analytical data (table 1) indicated that the perovskite concentrate contained 52.2 pct TiO2, 29.9 pct CaO, 7.2 pct FeO, 0.4 pct Cb2O5 (columbium pentoxide), 1 pct Ce, and other impurities.
The other sample, a high-grade concentrate from the Trail Ridge sand deposit in Florida, was representative of the ilmenite feedstock that is used in chloride-process TiO2 plants. The primary mineralogical components of this concentrate were rutile and altered ilmenite mixed with leucoxene. Also present were trace amounts of accessory staurolite, garnet, ferromagnesium silicate, apatite, and quartz minerals. The ilmenite sample contained 63.0 pct TiO2, 3.5 pct FeO, and 26.7 pct Fe2O3, along with minor amounts of other impurities, including calcium and magnesium oxides (table 1).
Preparation of TiC From Perovskite
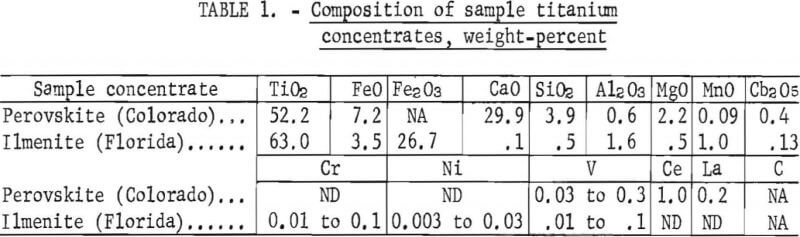
The initial laboratory tests to extract TiC from titanium-bearing minerals were conducted using a sample of perovskite. The high CaO content (29.9 pct) precluded treating perovskite by direct chlorination since CaCl2 accumulates as a liquid in the chlorination reactor and plugs the fluidized bed.
Preliminary thermodynamic data (figure 1), however, indicated that the perovskite could be reacted with carbon at approximately 3,050° F (1,950° K) to form a mixture of titanium and calcium carbides. The mixed carbides were subsequently contacted with H2O, which selectively decomposes CaC2 to hydrated lime and acetylene and simultaneously frees the TiC. The TiC was separated from the lime, dried, and treated with chlorine to produce TiCl4, as shown in the proposed flowsheet in figure 2.
In testing the initial concept described above, the Bureau used pelletized charges consisting of 10 pounds of perovskite concentrate (ground to minus 200 mesh) blended with carbon and 0.3 pound cereal binder. Pellets about 0.5 inch in diameter were formed on a rotating-disk pelletizer and dried at 255° F for 24 hours prior to carbiding. Water added to the blended constituents during pelletization was equivalent to 12 wt-pct of the charged perovskite.
Perovskite-carbon charges were fused in a 40-kw single-phase arc melting furnace. Carbiding was accomplished in a graphite crucible with an 8-inch ID, a wall thickness of 1 inch, and a height of 11 inches. A layer of minus 10-mesh charcoal was placed on the bottom of the reactor to serve as a working hearth.
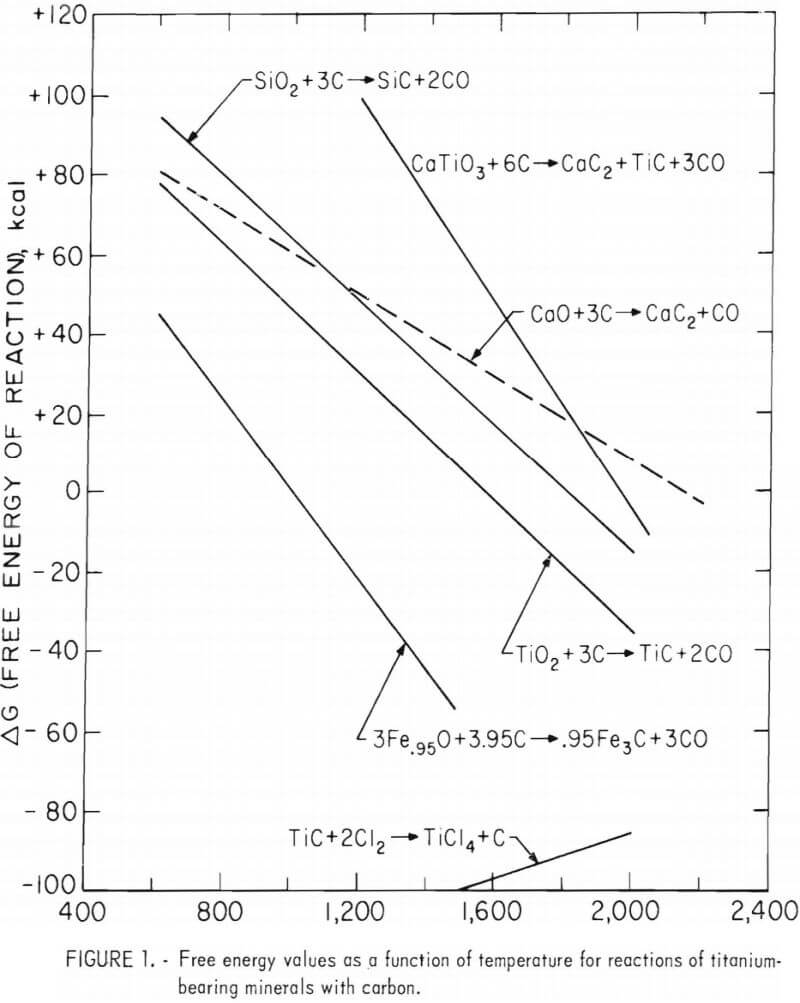
In a typical carbiding operation, sufficient charge was hand fed to form a pool between the two carbon electrodes (which were each 2 inches in diameter), and a vibratory feeder was then used to feed the pellets at a rate commensurate with their fusion rate. Temperatures, as measured with an optical pyrometer, averaged approximately 4,350° F in the electrode area and 3,270° F at the periphery of the fused material. After heating the charge for 3 hours, the fused product was covered with crushed charcoal and cooled overnight in the furnace.
Operating parameters in the three steps, carbiding, grinding, and TiC recovery, were studied. In the carbiding step, the effects of varying the ore-carbon ratio of the charge on the purity and recovery of TiC were investigated. In the second step, the effects of grinding and H2O leaching of the fused titanium and calcium carbides on the liberation of TiC were established. In the third step, which involved the separation of TiC from the H2O slurry, the effects of H2O elutriation and gravity concentration on the purity and recovery of the purified product were determined.
In discussing the results that were obtained for each of the three steps, the calcium impurity is reported as CaO, although the element existed as CaC2 in the reacted charges of the carbiding step and as Ca(OH)2 when the CaC2 was decomposed by H2O. This was done for reporting consistency.
Perovskite-to-Carbon Ratio
For a 10-pound charge of perovskite ore concentrate, the stoichiometric amount of carbon required to convert the titanium, calcium, iron, and silicon oxides to their respective carbides is 4.7 pounds. However, in a series of tests made with 4.25-, 5-, 6-, and 7-pound additions of carbon in the charges, the best carbide yields were achieved with charges that contained 7-pound additions and were reacted for 3 hours.
The impurity content of the recovered TiC depended on the proportion of carbon added to the furnace charge. The CaO content of the purified product decreased from 17.1 to 1.7 pct as the amount of carbon was increased from 4.25 to 7 pounds. Addition of the larger quantities of carbon enhanced the fusion of those charges that contained greater amounts of carbon dissolved in the TiC lattice (as compared with the charges that contained lesser amounts of dissolved carbon). Increasing the amount of carbon added to the charge also lowered the titanium content from 72.5 to 63.0 pct and increased titanium recovery from 71 to 72.7 pct in the purified product. Increasing the amount of added carbon also lowered the oxygen content of the purified TiC from 6 to 3.1 pct. Only 0.4 to 1.4 pct nitrogen was present in the purified TiC. Thus, these tests showed that the lowest CaO content (1.7 pct) in the purified TiC was obtained when a 10-pound perovskite charge was reacted with 7 pounds of carbon at 4,350° F for 3 hours.
Effect of Grinding on TiC Liberation
Microscopic examination of the carbided charges showed that the TiC crystals were embedded in a CaC2 matrix together with free graphite. Grinding the material was not sufficient to liberate the TiC, but liberation was accomplished by using H2O to decompose the matrix. The carbided material from the furnacing step was first reduced in a laboratory jaw crusher to minus 0.75 inch and then dry ground in a small rod mill to pass through a 35-mesh sieve. The carbided material was not wet ground because dry samples of the mixed carbides were needed for chemical analyses. Another reason for dry grinding was to avoid a dangerous buildup of acetylene in the rod mill. The ground, mixed carbides were sampled, and from the remaining portion a 2.2-pound charge was prepared for reaction with H2O. Since the CaC2 in the 35-mesh ground material reacted rapidly with H2O to achieve adequate liberation of the TiC, a finer grind was not necessary.
Separation of TiC from H2O Slurry
A series of tests established that 2 hours was sufficient time to contact the ground material with H2O, and that 0.26 gal of H2O for each 2.2 pounds of ground, mixed carbides was adequate to decompose the CaC2. Because CaC2 reacts vigorously with H2O, generating both heat and acetylene, the ground carbides were slowly stirred into the H2O in a beaker over a period of 30 min. The resultant slurry was stirred an additional 90 min and then ball milled in an 8-inch-diam mill for 3 min to break up the Ca(OH)2 lumps that had formed.
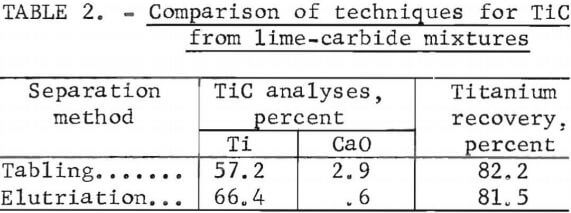
The purified TiC was separated from the residual impurities in the slurry, which included hydrated lime and graphite particles. Separation techniques that were tested included H2O elutriation and gravity concentration by tabling. As shown in table 2, elutriation resulted in a slightly better grade of TiC than did tabling, from the standpoint of CaO content. A sample of purified TiC obtained by elutriation contained only 0.6 pct CaO, compared to 2.9 pct CaO in the product obtained by tabling.
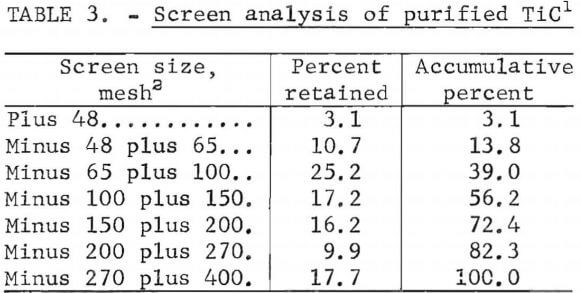
For the elutriation test, the slurry was fed into a column that was used to wash the TiC (plus 400-mesh fraction) free of solid impurities with a rising H2O flow. This glass column was about 54 inches high with a 3.5-inch ID and contained a stainless steel screen in its base to retain the course carbide. The slurry was fed into the upper portion of the column while H2O flowed at a rate of 0.21 gal/min into the bottom below the screen. This washing classified the TiC product into two fractions, with 80 pct of the titanium recovered in a plus 400-mesh fraction (the underflow) and the remaining 20 pct of the titanium contained in the minus 400-mesh fraction (the overflow). Size distribution data for the plus 400-mesh fraction of purified TiC (table 3) indicate that 39 wt-pct of this fraction was plus 100 mesh and 72.4 wt-pct was plus 200 mesh. Subsequent testing discussed later in this report showed that the overall titanium recovery in the purified product can be increased to 93 pct by recovering the minus 400-mesh TiC from the overflow stream.
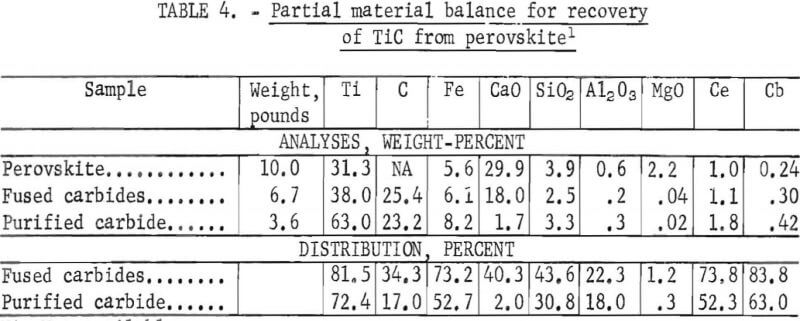
A partial material balance for the recovery of purified TiC from a perovskite sample is shown in table 4. The data show that 72.4 pct of the titanium in the perovskite charge was recovered as a purified product which analyzed, in wt-pct: 63.0 Ti, 23.2 C, 1.8 Ce, 0.4 Cb, and 1.7 CaO, plus other impurities. During the carbiding step, several constituents of the perovskite charge were emitted with the furnace offgases. Percentages of constituents lost during carbiding were 18.5 Ti, 26.2 Ce, 16.2 Cb, 56.4 Si, 77.7 Al, 98.8 Mg, and 55.7 CaO. The tailings from the purification step contained only 8 pct of the titanium, but also contained 32 pct of the cerium and 11 pct of the columbium. The recoveries of cerium and columbium-two valuable byproducts of the purified TiC product-were 52.3 and 63 pct, respectively. In practice, the overall recoveries of these valuable constituents could be increased by treating the dust captured from the furnace offgas and the tailings from the TiC purification.
Preparation of TiC from Ilmenite
Titanium carbide can be made directly from ilmenite, but the crystalline product contains impurities from the ilmenite ore and the unreacted charge. Pyrometallurgical treatment of ilmenite in an electric arc furnace to recover TiC is best carried out using a two-stage procedure. In the first stage, the iron oxides in ilmenite are carbothermically reduced to molten iron (at 2,820° to 2,960° F) and separated, leaving titanium as a CaTiO3 slag. Lime is added with the ilmenite charge to obtain a slag of high fluidity and low iron content. In the second stage, titanate slag-coke-lime charges are converted at a higher temperature (about 4,350° F) to a mixture of titanium and calcium carbides.
Laboratory Tests
Initial carbiding tests on titanium slag were performed on a high-TiO2- content sample made from ilmenite that was reduced without using a lime additive. This 50-pound slag sample was obtained from an industrial source, and its composition is shown in table 5. For the laboratory test, 10 pounds of this slag was ground to pass through a 35-mesh screen and mixed with metallurgical-grade lime and carbon to yield a charge analyzing 21.4 pct Ti, 31.5 pct CaO, and 44.7 pct C. Lime additive was used to test the concept of substituting a mixture of titanium slag and lime for perovskite in producing TiC from sources other than rutile. In practice, the lime would be added with the ilmenite charge to obtain a titanium slag that is low in FeO content (<3 pct Fe) and fluid enough to be removed from the reduction furnace.
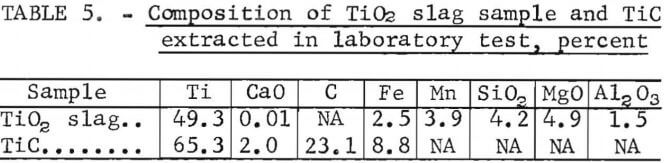
Pelletized constituents blended with 2 wt-pct cereal binder were heated to a temperature of 4,350° F (measured on the surface), and the products were treated in the same manner as was the perovskite material. About 80 pct of the titanium in the slag charge was recovered as purified TiC containing 65.3 pct Ti, 23.1 pct C, and 2.0 pct CaO, plus other impurities (table 5). The product, which was dried at 195° F, consisted of TiC and graphite as primary phases and Ca(OH)2 as a barely detectable phase. These tests demonstrated that titanium slag with a high lime content can be converted to TiC with results similar to those achieved with perovskite. Best conversions of titanium slag to carbide (95 percent) were achieved when 10-pound slag charges were reacted with 14.3 pounds of carbon and 8.6 pounds of lime at 4,350° F for 3 hours.
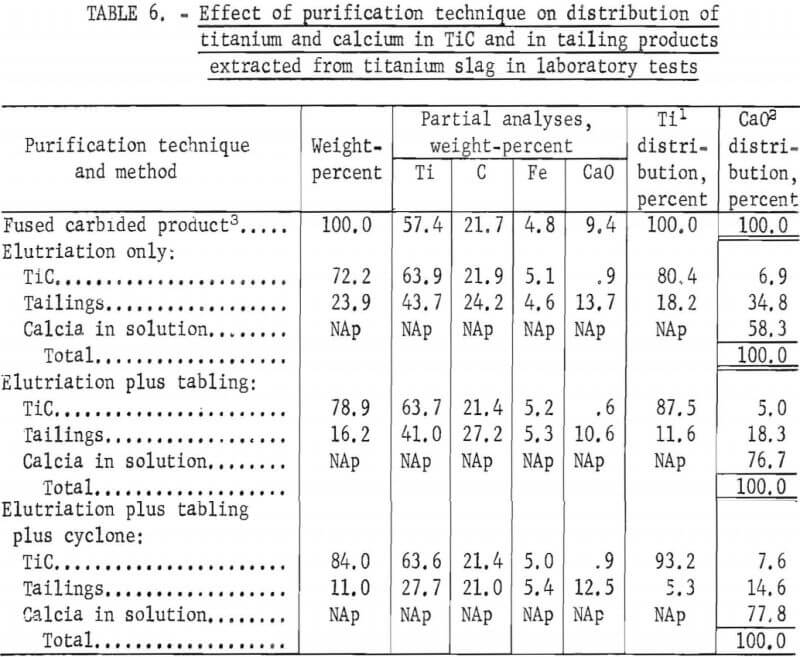
Additional tests on the purification of TiC made from titanium slag showed that use of a three-step procedure can increase the overall titanium recovery to 93.2 pct (table 6). In comparison, the elutriation step alone recovered only 80.4 pct of the titanium as purified carbide in the underflow. The second step, tabling the minus 400-mesh carbide from the overflow, recovered additional TiC and increased the titanium recovery to 87.5 pct. The third purification step, passing the table tailings through a hydrocyclone, further increased the amount of titanium recovered from the fused titanium and calcium carbides to 93.2 pct.
Table 6 also summarizes CaO distribution in the products of three-step TiC purification. A total volume of 26.42 gal of H2O was used in the three steps, and 78 pct of the calcium separated from the carbided products was dissolved in H2O; about 8 pct of the calcium was left in the purified TiC, and about 14 pct remained in the tailing.
Results of a series of tests showed that dilute HCl leaching of the TiC at room temperature for 1 hour removed about 70 pct of the residual CaO left in the purified product. However, this investigation was not pursued because acid leaching could present a waste acid disposal problem in a conceptual plant. In devising a flow sheet for preparing chlorination feedstock, one of the goals was to avoid using acid leaching techniques.
Larger Scale Tests
In a larger scale furnace, a two-stage procedure was employed to produce TiC from an ilmenite starting material. First, ilmenite concentrate was reacted with coke and lime, which separated 95 pct of the iron as a pig iron and left the titanium as a CaTiO3 slag with a low iron content (2 pct FeO). Second, the slag mixed with carbon and additional lime was reheated, yielding a mixture of titanium and calcium carbides from which purified TiC was recovered.
A statistically designed test series was used to study the effect of charge composition on the grade and recovery of TiC from the intermediate slag product. Unfortunately, this work was terminated in the early phase of the larger scale test program (due to changing research priorities), but the results of one completed test series suggest that using an electric arc furnace to carbide the charges limits the scale of operation and consumes a large quantity of energy. In some instances, carbiding of 250-pound charges resulted in the fusion of only about two-thirds of the material near the electrodes while the other third near the crucible wall remained unfused and was not converted to carbide. One possibility for reducing energy consumption is to prereduce the ilmenite-coke-lime charge in a gas-fired rotary kiln; the hot material could then be melted down in an electric arc furnace to separate the iron from the titanium slag. An alternate continuous carbiding method that may offer possibilities for lower energy consumption is the use of an induction-heated shaft furnace in which pelletized charges are fed into the top and the products are withdrawn from the bottom and cooled in an inert atmosphere (CO produced from carbiding). Conversion of perovskite or titanium slag to carbide could be conducted in a similar manner to save energy.
Preparation of Titanium Slag
About 950 pounds of titanium slag from a Florida ilmenite concentrate that was made from a beach sand deposit was prepared for use in larger scale carbiding tests. When this slag was prepared, only the high-grade ilmenite sample was available in sufficient quantity for these tests, A lower grade ilmenite concentrate from the more abundant domestic rock deposits would have been the preferred starting material, but such material was not available.
To prepare the slag, 1,000 pounds of the ilmenite sample of the composition shown in table 1 was blended with 160 pounds of metallurgical-grade pebble lime (flux) and 78 pounds of coke. The blended charge was fed into a three-phase arc furnace and reacted at about 2,820° F to yield a fluid CaTiO3 slag containing 36.6 wt-pct Ti, 1.4 wt-pct FeO, and 22.1 wt-pct CaO. About 30 pounds of foundry carbide added to the bath completed the reduction step, after which the molten iron and slag products were tapped separately. About 90 pct of the titanium contained in the ilmenite charge was recovered as slag; the remainder was removed with the particulates in the furnace offgas. Also, 5.5 pct of the iron in the ilmenite charge was left in the slag, 89 pct of the iron was separated, and 5.5 pct was lost as particulate in the furnace offgas and during handling of the charge and products.
Carbiding of Titanium Slag
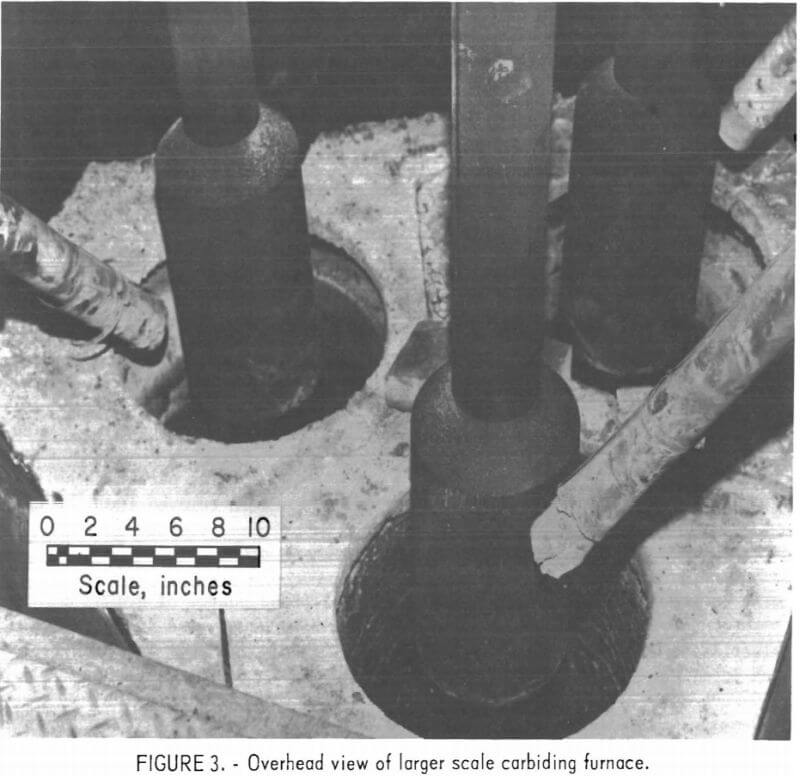
The larger scale carbiding furnace consisted of three rammed-carbon crucibles, Each crucible was 36 inches high and had a 16-inch ID, walls 2 inches thick, and a 3-inch-thick graphite bottom. The three crucibles were positioned at the vertices of an equilateral triangle on a water-cooled grounding plate, directly under the three carbon electrodes of a three-phase smelting furnace. A triangular steel shell was placed around the crucible assembly, and crushed charcoal was poured into the voids between the crucibles and the steel shell. An overhead view of the assembled furnace is shown in figure 3. Electric power was supplied to the furnace by a 1,000-kva transformer.
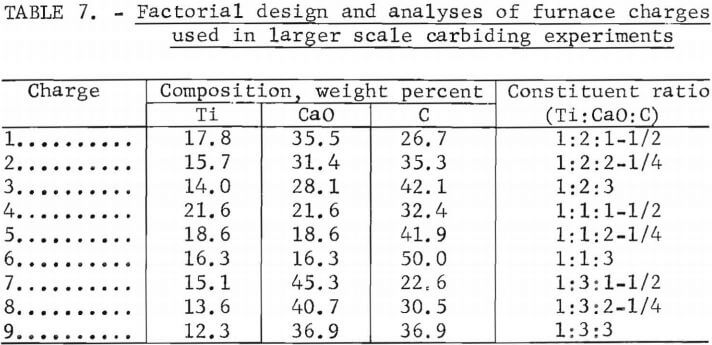
A factorially designed series of nine tests was conducted to study the effect of charge composition on the grade and recovery of TiC from the slag starting material. Charges composed of titanium slag blended with additional lime and carbon were prepared using composition regions suggested by previous
laboratory results. The charges were pelletized (into plus ¼- minus 3/8-inch- diam balls) to facilitate fusion and reduce dust loss from the furnace. Samples of the pelletized charges were analyzed, and the results are shown in table 7.
 About 120 pounds of pelletized charge was added to each crucible by charging through individual chutes that extended down through the furnace roof. A variable-speed belt feeder fed the charge at an average rate of 2.7 lb/min. After 180 min, the power was turned off, the electrodes were raised, and the fused products were covered with crushed charcoal prior to cooling. Energy consumption averaged 3 kwhr/lb of charge, or 1,080 kwhr for each run. A typical section of a fused charge is shown in figure 4.
About 120 pounds of pelletized charge was added to each crucible by charging through individual chutes that extended down through the furnace roof. A variable-speed belt feeder fed the charge at an average rate of 2.7 lb/min. After 180 min, the power was turned off, the electrodes were raised, and the fused products were covered with crushed charcoal prior to cooling. Energy consumption averaged 3 kwhr/lb of charge, or 1,080 kwhr for each run. A typical section of a fused charge is shown in figure 4.
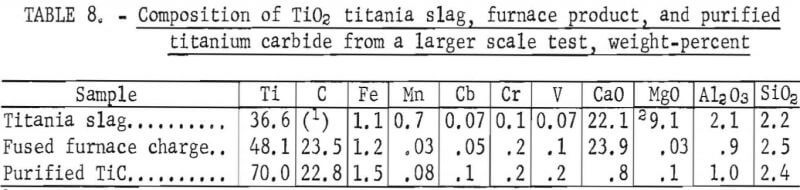
The carbided charges were removed from the crucibles, reduced in a jaw crusher to minus 0.75 inch in size, dry ground in a rod mill to minus 35 mesh, and sampled. Each 2.2-pound charge of ground carbides was leached in 0.26 gal of H2O, and the resulting slurry was subjected to H2O elutriation to recover purified TiC. The procedure employed for elutriation was identical to that used in the small-scale tests except that only the plus 400-mesh material was recovered as product from the underflow; the minus 400-mesh TiC was left unrecovered in a tailing fraction (the overflow). The best grade of TiC product contained 70 pct Ti, 22.8 pct C, and 0.8 pct CaO, with 60.7 pct of the titanium recovered from a charge that analyzed 13. 6 pct Ti, 30.5 pct C, and 40.7 pct CaO (table 8).
The results of the factorially designed larger scale test series are summarized in figures 5 through 8, which show the effects of charge composition on the titanium and calcia contents of purified TiC and on the percent of titanium recovered. Statistically, no significant effects could be verified, but there appeared to be a significant interaction between the
variables. The project was terminated, however, before the experiment could be repeated to evaluate this interaction. In general, these results suggest that increasing the proportions of carbon and lime in the charges lowers the CaO and titanium contents of the purified product. These tests also showed that the carbon content of the purified TiC increased from 14.6 to 50.6 pct as the amount of carbon that was added to the charge was increased from 22.6 to 50.0 wt-pct.
Chlorination of TiC
Chlorination of TiC to produce TiCl4 is based on the following equation:
TiC + 2Cl2 → TiCl4 + C………………….(3)
The free energy value for the reaction of TiC with elemental chlorine (fig. 1) is favorable for chlorination to proceed at ambient temperatures.
To determine the minimum temperature at which the above reaction occurs, a 20-gram charge of TiC was placed in a 1-inch-diam furnace and contacted with a flow of chlorine gas. Ignition occurred at about 435° F, as indicated by a thermocouple placed in the charge, The charge temperature increased rapidly to 1,310° F.
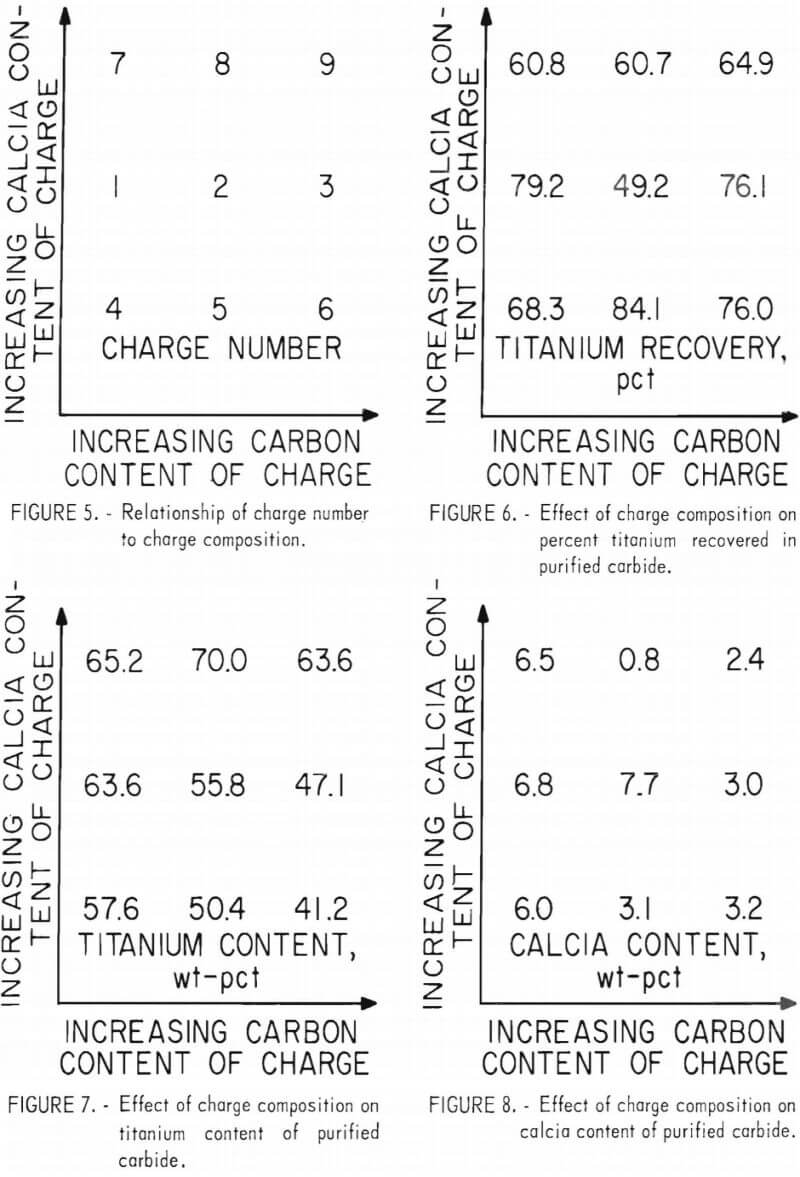
The approximate reaction rate of TiC chlorination was determined in a series of static-bed tests in which a 0.5-gram charge was contacted with a mixed gas flow of 20 pct chlorine and 80 vol-pct argon. The log of TiC weight loss in 15 min is plotted against the reciprocal of temperature in degrees absolute and against temperature in degrees Fahrenheit in figure 9. (The dotted portion of the plot represents an estimation based on only one point in a typical Arrhenius treatment of a chemically controlled reaction; the solid line illustrates the actual experimental data.) The data indicate that the reaction rate in these experiments was controlled by chemical kinetics from 575° to 650° F. Above 650° F, the reaction rate was limited by the diffusion of chlorine to the reacting surfaces of TiC.
To study the effect of chlorination temperature on the extraction of titanium from TiC, a series of tests was performed in a vertical 1-inch-diam furnace. In these tests, a mixed flow of chlorine (at 350 cc/min) and argon (at 150 cc/min) gases was contacted with TiC (minus 10- plus 200-mesh material) in a static bed maintained at temperatures of 570° to 1,020° F. The TiCl4 product was vaporized from the reactor and collected in a water-cooled condenser as a liquid. The residue left in the chlorination reactor was analyzed to determine the percent of titanium that was extracted. The results indicated that only small amounts of the TiCl4 product were collected at chlorination temperatures of 570° to 820° F. At temperatures of 940° F and above, the reaction was essentially complete, with 98.8 pct of the titanium contained in the TiC extracted as TiCi4.
Subsequent tests were conducted to determine the response of TiC to chlorination in two other types of reactors, a fluidized-bed chlorinator and a fused-salt bath. Because the chlorination reaction is highly exothermic,
close control of temperature is necessary to selectively chlorinate TiC in the presence of associated impurities (principally calcium and, to a lesser extent, iron, aluminum, and silicon). A partial analysis of the TiC sample made from ilmenite slag and used in these experiments is as follows, in percent: 70.6 Ti, 23.2 C, 1.7 Fe, 1.1 CaO, 1.3 SiO2, and 0.8 Al2O3.
Fluidized-Bed Reactor
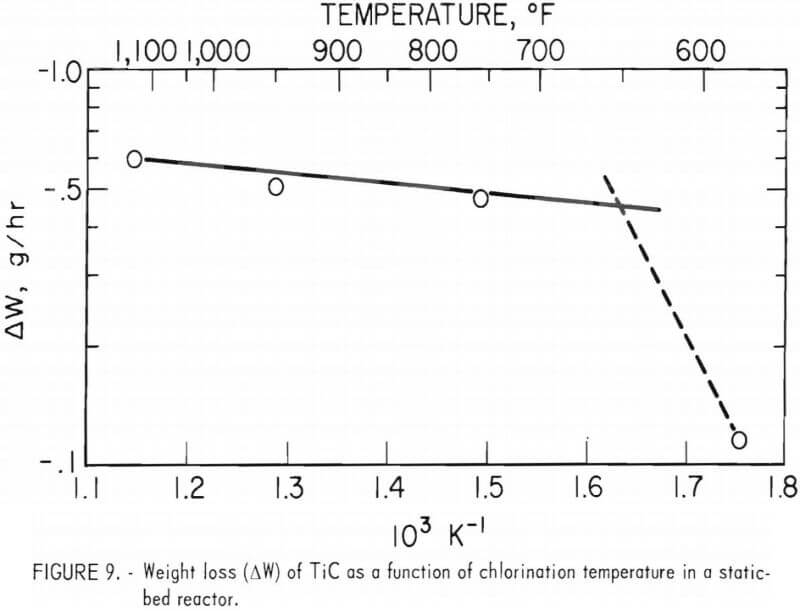
A 2-inch-ID Vycor tube was employed as the fluidized-bed reactor. Chlorination temperature was controlled by using argon gas as a coolant, at a constant rate of 2 l/min, and by varying the chlorine gas flow rate from 0.5 to 3.5 l/min, depending on the desired reaction temperature. Only two sized fractions of the carbide sample were chlorinated-the minus 100- plus 200-mesh and the minus 200- plus 325-mesh fractions.
The relationship between chlorine flow rate and reaction temperature is shown in figure 10. A chlorine flow rate of 2.1 l/min produced a reaction temperature of 940° F, and about 2.8 g/min of TiC was chlorinated (from the minus 100- plus 200-mesh fraction). The reason that chlorination of the coarser carbide fraction yielded a higher reaction temperature than did the finer carbide may be explained as follows: The heat generated was solely dependent on the chlorine flow rate (assuming that all the chlorine reacted), and the hot, finer-sized TiC particles transferred more heat to the gas stream; thus, more heat left with the exiting gases.
Tests made in the fluidized-bed reactor showed that TiC chlorinated to a greater degree than did the associated impurities. For instance, at 840° F, 98 pct of the titanium contained in the TiC that was in the minus 100- plus 200-mesh fraction was converted to TiCl4, while 76 pct of the iron and 37 pct of the CaO and MgO impurities chlorinated.
Fused-Salt Bath Reactor
A series of preliminary tests was conducted to examine the possibility of using a fused-salt reactor to treat TiC with chlorine. In a reactor of this type, the fused salts would transfer the heat generated by the highly exothermic carbide chlorination reaction to the walls of the air-cooled reactor. Besides transferring heat, the fused salts would also react with the iron and aluminum chlorides to form stable salt complexes that would remain dissolved in the bath while the purified TiCl4 vaporized.
A 1-gram charge of minus 325-mesh TiC was fed into a bath composed of a 1:1 mole ratio of MgCl2 and NaCl salts in a reactor operated at 930° to 1,020° F. The reactor, made of 4-inch-ID glass tubing, was 6 inches high, and had a sidearm through which the carbide feed was introduced in 0.1-gram increments.
Tests in the fused-salt bath reactor showed that at 1,020° F, approximately 88 pct of the titanium in the carbide feed was converted to high-purity TiCl4, which volatilized and collected in an adjacent water-cooled condenser.
Donflestic Titaniferous Ores
A carbiding procedure was devised that enables production of TiCl4 from perovskite and other titaniferous materials containing appreciable quantities of CaO. The principal operations involved are:
- Selectively reducing the iron oxides in ilmenite to separate the iron and prepare a CaTiO3 slag of low iron content
- Converting either the titanate slag or perovskite concentrate to a mixture of titanium and calcium carbides and selectively extracting TiC by decomposing CaC2 in H2O, and
- Chlorinating purified TiC to produce TiCl4.
Tests performed in laboratory and larger scale electric arc furnaces demonstrated that a high reaction temperature of approximately 4,350° F is necessary to achieve carbide conversion in charges reacted for 3 hours. Up to 95 pct of the titanium in the perovskite and ilmenite slag charges, which contained 36 to 50 pct carbon and 24.7 to 48 pct CaO, was converted to mixed carbides.
Calcium carbide in the mixed carbides was selectively decomposed to hydrated lime and acetylene by reaction with H2O, and this reaction concurrently liberated the unreacted TiC. The resulting hydrated lime was separated from the TiC by dissolution in H2O and by physical methods, including elutriation and gravity separation.
The reaction of purified TiC with chlorine gas was initiated at temperatures as low as 435° F, followed by a rapid increase to 1,310° F. Tests conducted in a static-bed reactor, using a flow of 20 pct chlorine and 80 vol-pct argon, showed that the reaction rate was controlled by chemical kinetics from 575° to 650° F. Above 650° F, the reaction rate was limited by the diffusion of chlorine to the reacting surfaces of the TiC. In subsequent tests using a fluidized-bed reactor, the chlorine flow was varied while the argon flow was held constant. In these tests, 98 pct of the titanium in the TiC was extracted at 840° F.
The Bureau of Mines conducted laboratory and larger scale studies on samples of domestic perovskite and ilmenite ore concentrates to devise a procedure for recovering TiC from these ores. From the concentrates, mixtures of titanium and calcium carbides were produced. The carbides were then ground and reacted with water. This reaction decomposed the CaC2 to acetylene and hydrated lime and simultaneously liberated the TiC.
Using a combination of elutriation and gravity-separation techniques to separate the hydrated lime, approximately 93 pct of the titanium was subsequently recovered as purified carbide containing 54 to 70 pct titanium with CaO content as low as 0.6 pct. This combination of techniques can be used to separate more than 90 pct of the CaO from the TiC. Therefore, this carbiding procedure is a viable method for treating titaniferous materials, including perovskite, that contain more than 20 pct CaO.
Tests made in a static-bed reactor showed that for the reaction of TiC with elemental chlorine to produce titanium tetrachloride (TiCl4), a temperature of at least 940° F is desirable to achieve a suitable extraction of titanium in a laboratory chlorination reactor. In fluidized-bed tests at 840° F, 98 pct of the titanium contained in TiC was extracted.
