Table of Contents
A preliminary series of laboratory flotation tests was performed to determine the nature of the unleached gold values of the CIL tailings. The gold in the Mercur mining district has been identified historically as being associated with sulfide and carbonaceous minerals in addition to a percentage occurring as free gold. Test results indicated that higher gold-containing concentrates could be produced by the use of reagents designed for flotation of the sulfide mineral fraction. Acid digestion tests of certain CIL tailings samples indicated that approximately 62 pct of the gold was present in the free state or associated with the sulfide minerals. About 20 pct of the gold was characterized as being associated with the carbonaceous material while about 17 pct was present with the silicas.
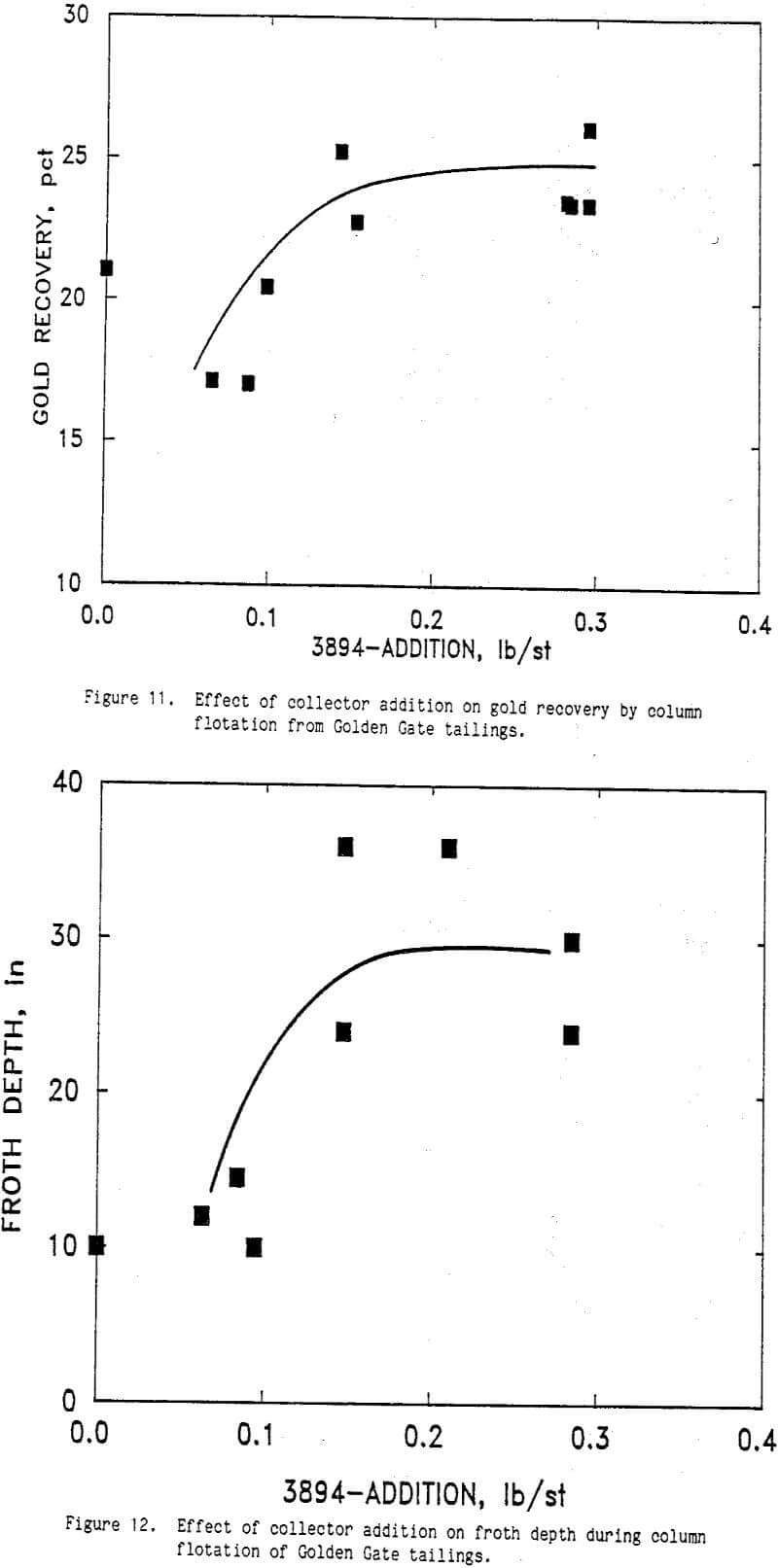
Column Flotation
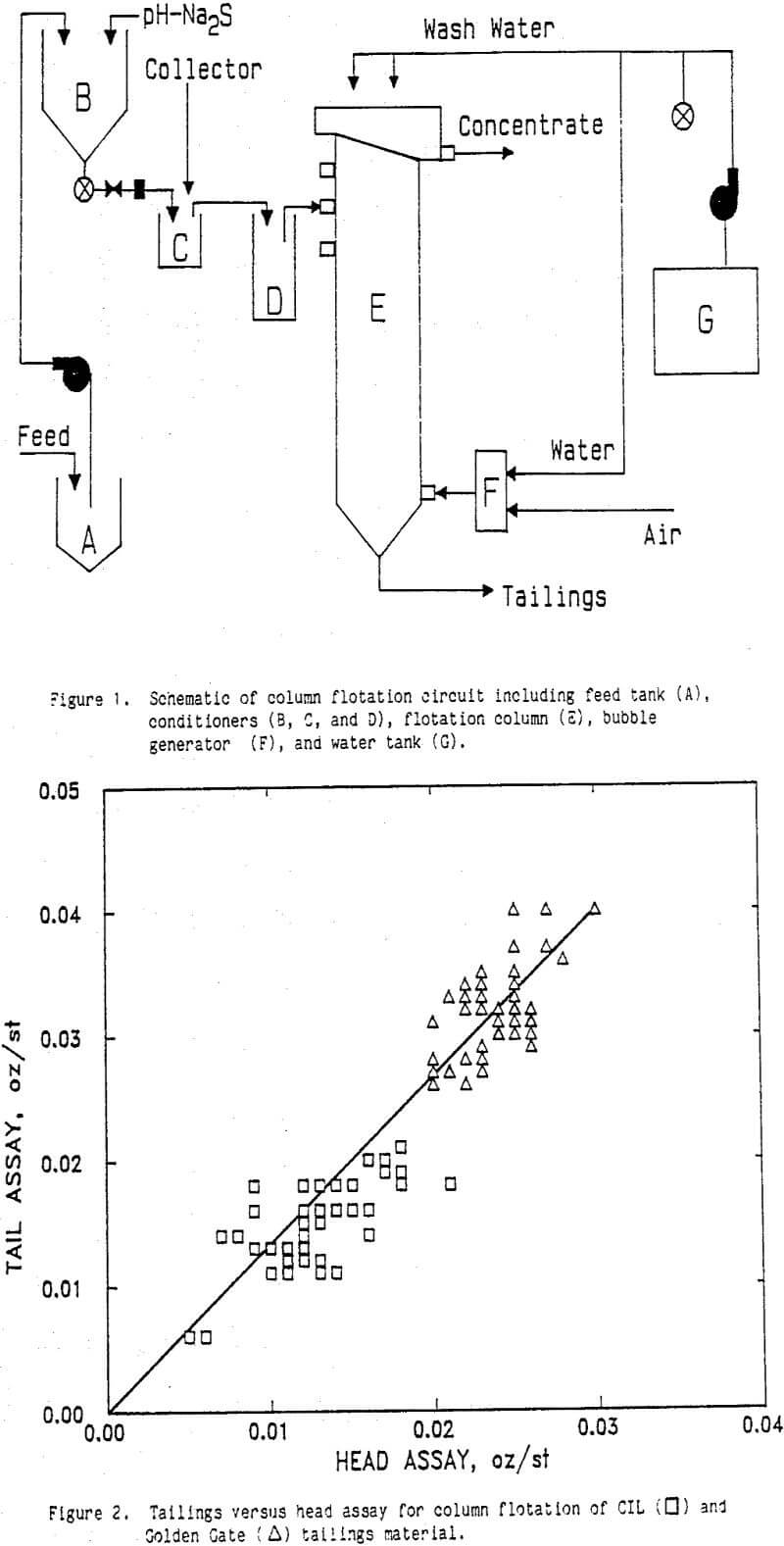
The feed to the scavenger column flotation unit was a portion of the plant operations CIL tailings which ranged from 0.01 to 0.02 oz Au/st in unleached gold values. There was a direct correlation between the head grade and the floatable gold in the tailings as is shown in Figure 2. Data are included from various flotation tests, so the linear relationship between head and tailings assays suggests a lack of control over the flotation parameters. That is, 20 to 30 pct of the gold was floatable under the investigated conditions irrespective of which parameters were varied. However, some general trends were observed within short test series and are discussed in the following sections.
Effect of Collector Addition
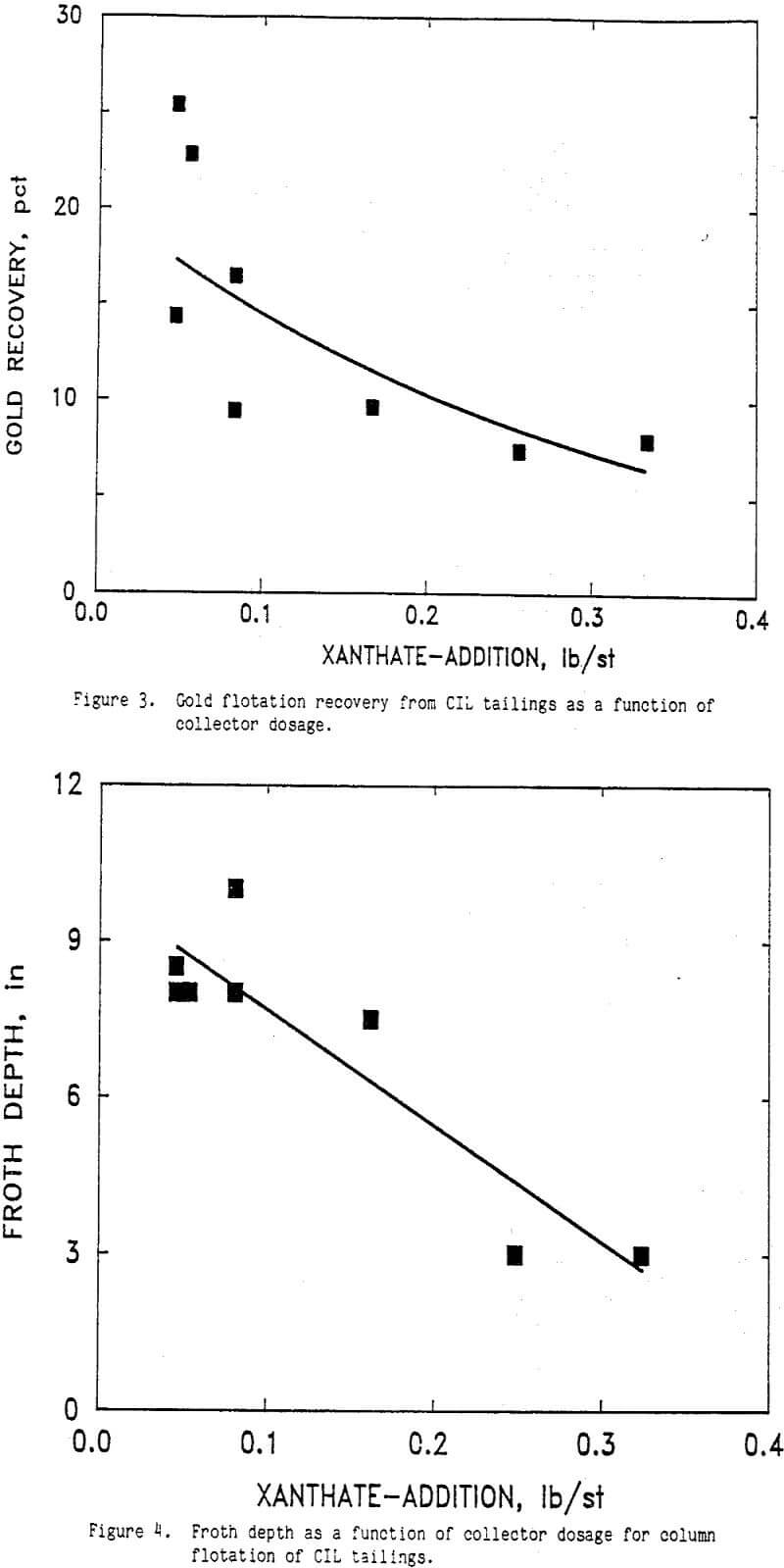
The effect of AERO 350 xanthate additions is shown in Figures 3 and 1 where gold recovery and froth depth are plotted respectively as a function of collector dosage. Other conditions during this test series were 0.10 lb Na2S/st, 0.085 to 0.1 lb CuSO4/st, and 1.33 lb AERO 3177/st. The pH of the CIL tailings slurry ranged from 10.6 to 11.1 and averaged 10.8. A froth wash water rate of 1.9 to 2.2 L/min was used during these tests.
Gold recovery decreased with increasing AERO 350 dosage (Figure 3). In addition, the froth depth of the column decreased with increasing AERO 350 dosage (Figure 1). Decreased gold recovery was observed with increased collector addition indicating that an overdose of collector was detrimental to flotation. The collector dosage affected the froth level possibly through interactions with other surface active agents present in the solutions.
Effect of pH and Redox Potential
The effects of pH and sodium sulfide addition on gold recovery and on the redox potential of a gold electrode were measured. Correlations between recovery, pH, and redox potentials might be used in a control scheme to regulate reagent additions. The feed rate to the column was maintained at 10 gal/min at approximately 40 pct solids by weight. Other reagent dosages were 0.1 lb CuSO4/st, 60 mL diesel oil/st, 1.18 lb AERO 3177/st, and 0.056 lb AERO 350 xanthate/st. AERO 65 frother was added at 10 ppm to the bubble generator water. Column froth depth was maintained at 10 to 11 in for all tests in this series.
Recovery is shown as a function of pH and Na2S addition in Figure 5. Recovery decreased with a shift in pH from 9.5 to 11.5, while the effect of Na2S was more complex. At pH 9.5, recovery decreased with Na2S addition up to 0.13 lb/st but did not change with higher dosages. Under more alkaline conditions, recovery increased slightly with Na2S addition, and maximum recovery was observed at pH 11.5 with 0.18 lb/st.
The time-averaged redox potential of a gold electrode as a function of pH and Na2S addition is shown in Figure 6. Gold potentials decreased with increasing Na2S additions which is typical behavior for additions of a reducing agent such as Na2S. When no Na2S was added, potentials increased approximately 0.035 V per pH unit. Gold recovery is plotted in Figure 7 as a function of pH and redox potential. Local maxima can be observed at each pH level at potentials of about minus 0.31 V versus the silver-silver chloride (Ag:AgCl) reference electrode. A general increase in recovery is also observed as pH was varied from 11.5 to 9.5.
These results indicate that pH could be coupled with redox potentials in a control scheme to regulate reagent additions which would yield maximum flotation recovery. Although optimal recovery conditions are indicated in this surface plot at pH 9.5, concentrate grade decreased as pH was varied from 11.5 to 9.5. Therefore, a desired cut-off grade would have to be selected to maximize separation efficiency.
Autoclave-CIL
Extraction of gold from the flotation concentrate is an essential step in the successful recovery of unleached values from the CIL tailings. The recent addition of a pressure oxidation circuit at Mercur provided a potentially viable treatment option that enhanced the prospect of flotation as a means of recovering gold which is lost to the plant tailings.
Bench flotation tests were used to accumulate concentrate for CIL and autoclave-CIL tests. As was expected, the recoveries from direct leaching ranged from pct. However, recoveries ranged from pct when leaching was performed after pressure oxidation at 225° C and 420 psi.
During pilot plant flotation tests concentrate was available for autoclave-CIL tests to evaluate recoveries. However, recoveries after pressure oxidation were inconsistent, and various pretreatment procedures were examined to try to stabilize or improve recovery. Washing the concentrate or washing, filtering, drying, and repulping improved recovery in most cases. Flotation reagent residues were initially suspected of having an adverse effect on recovery. However, leach tests conducted on concentrate doped with flotation reagents gave similar results to tests using concentrate without reagents. Native carbonaceous material or the undersize carbon residues from the leach circuit were also considered as probable causes of inconsistent leach recoveries, but neither has been confirmed or refuted as of this writing.
The autoclave product was treated with BaO2, PbO, or H2O2 as polarizers and NaOCl as an oxidizer prior to leaching but no improvement in recovery was observed. The addition of NaOCl to the concentrate prior to autoclaving did show a minor increase in recovery. This treatment was not pursued because of the potential adverse effect of high chloride levels being introduced in the plant autoclave circuit.
 |
 |
 |
 |