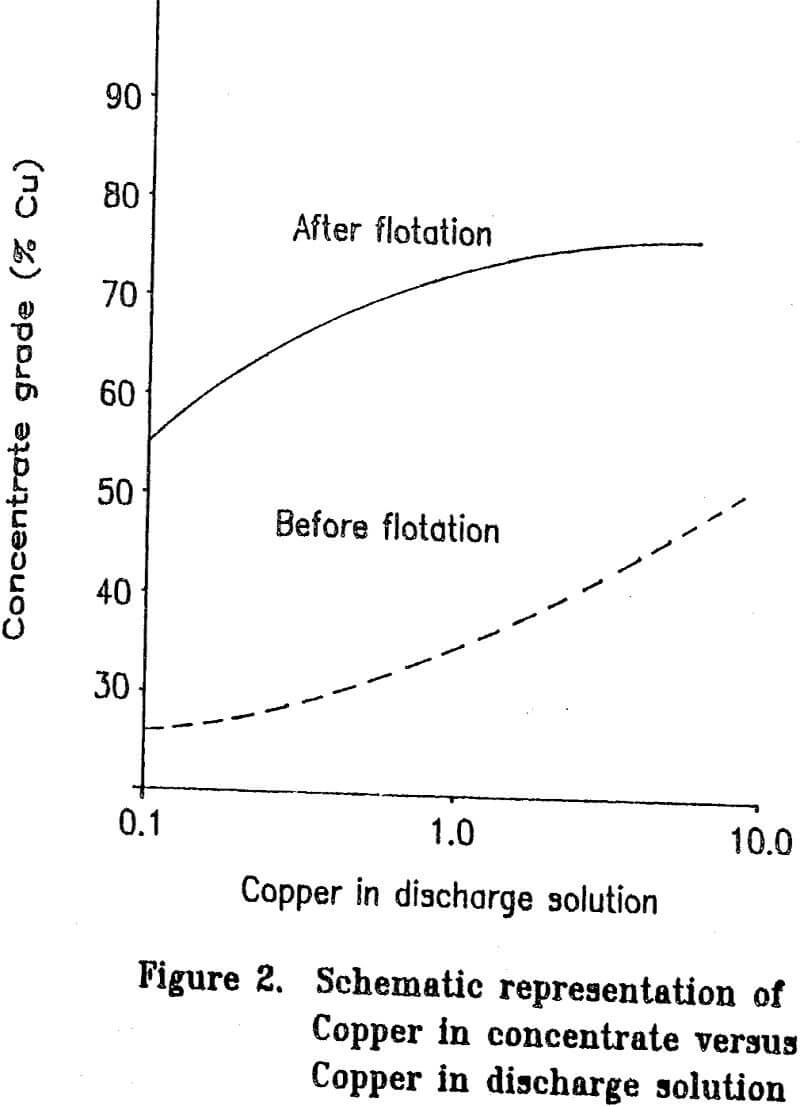
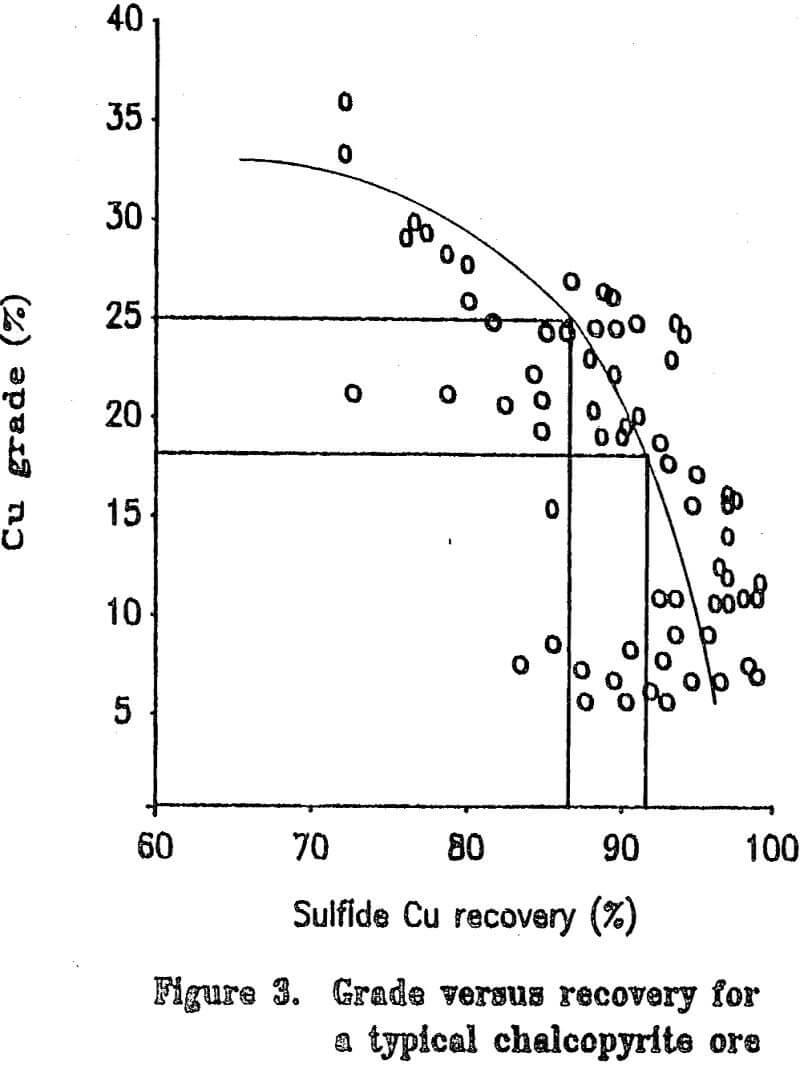
Most copper ore currently being mined contains copper mainly as chalcopyrite. Since chalcopyrite by itself is only about 35 percent copper, it is difficult to upgrade a concentrate by flotation to more than about 25 to 28 percent copper without losing a significant amount of copper during the cleaning steps. Some ores have associated problems such as chalcocite-coated pyrite and are difficult to upgrade to even 20 percent copper. The cost of shipping and smelting copper concentrates would be greatly reduced if the copper grade of the concentrate could be increased. If the grades of the concentrates are to be upgraded significantly, the mineral form of the copper must be chemically changed.
Process Chemistry
Depending on the conditions chosen, one of two idealized overall reactions would take place.
CuFeS2(s) + 2O2(g) → CuS(s) + FeSO4(aq)…………………………………..(1)
2CuFeS2(s) + 11/2 O2(g) + H2O → Cu2S(s) + 2FeSO4(aq) + H2SO4(aq)…………………..(2)
8 ½ O2(g) + 2H2O(1) + 2CuFeS2(s) → 2CuSO4(aq) + Fe2O3(s) + 2H2SO4(aq)………………..(3)
2O2(g) + CuS(s) → CuSO4(aq)…………………………………………………….(4)
Autoclave temperature would also affect the final concentration but it is necessary to limit the reaction temperature in the reduction autoclave to less than about 220°C because copper will start to react with pyrite by known secondary enrichment reactions to form chalcocite- coated pyrite. This would interfere with subsequent separation of a high-grade concentrate by flotation.
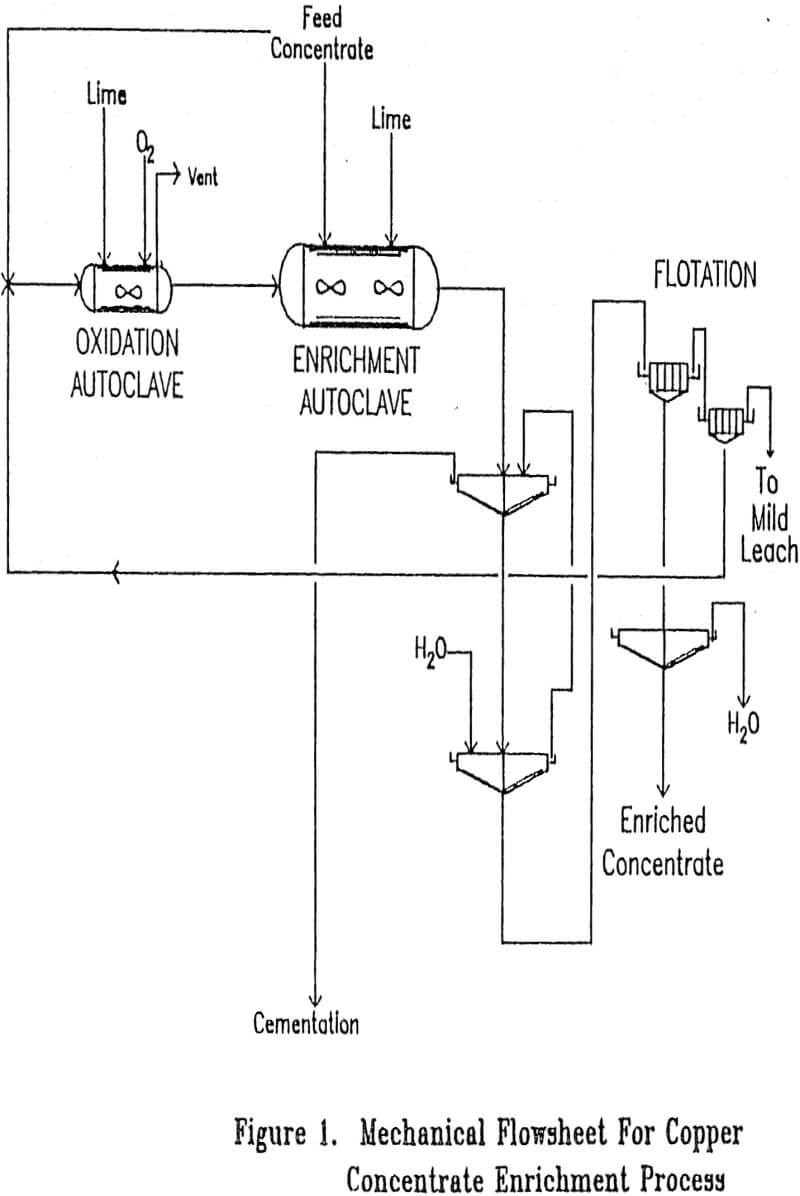
Frequently when a process operates with very low losses under normal operating conditions, a significant fraction of the total loss will occur during relatively minor upsets. Significant losses could occur from upsets, such as loss of temperature control on the enrichment autoclave or loss of concentrate flow into the enrichment autoclave, since such upsets would result in dissolved copper being discharged from the enrichment autoclave. For that reason it is proposed that the solution discharged from the solid-liquid separation step be sent to cementation to recover lost copper after partial neutralization with lime so as to limit iron consumption.
Process Implications
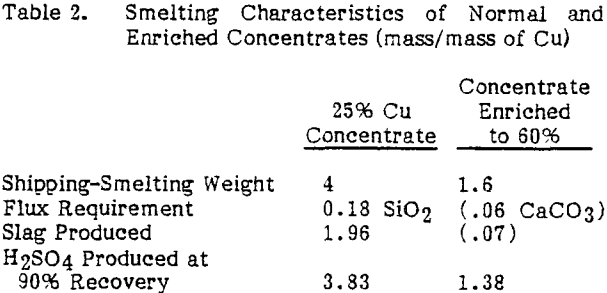
An important process requirement is that the enriched concentrates must be acceptable to existing smelters. Although enriched concentrates would affect each type of smelter in a slightly different way, there are some basic differences between smelting enriched concentrates and normal concentrates. The amount of sulfuric acid produced is reduced by about two-thirds and the slag produced is also reduced significantly. The values given for slag production and flux requirement assume that the enriched concentrate and the 25 percent concentrate are smelted so as to produce the same matte grade.
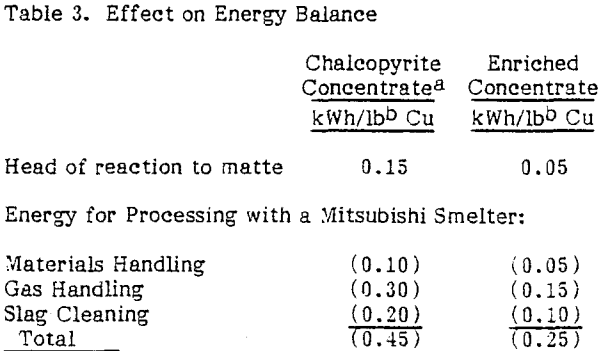
An additional consideration in treating such a high-grade concentrate is the effect that removal of sulfur and iron would have on the energy balance since oxidation of iron and sulfur provides most of the energy required for smelting. With the enriched concentrates the weight of material shipped and fed to the smelter will be reduced by about 60 percent. The incoming air and off-gas volumes and slag produced will also be reduced by 60 percent or more.
If it is assumed that smelting and shipping charges are proportional to the weight of the concentrate, the potential savings for shipping and smelting enriched concentrates when compared to normal concentrates would be about $0.13 to $0.16/lb ($0.29 to $0.35/kg) of copper.