Table of Contents
One objective of this study was to determine the critical parameters needed for designing of a plant to remove selenium from the mine leachate. While development of a full-scale engineering design was beyond the scope of the present study, the following design elements were evaluated:
A) Loading of the Alumina to the Adsorbers
B) Backwashing the Alumina Adsorbers.
C) Selenium and Silica Adsorption Capacities.
D) Hydraulic Loading and Empty Bed Contact Time.
E) Regeneration and Reloading of the Spent Alumina.
The development of design parameters was based on the use of adsorbers having configurations similar to those typically used for conventional water and wastewater filtration. Either gravity or pressure configurations may be used, although gravity configurations seem to provide better control of backwash and regeneration conditions and very likely will be the least-cost alternative. In all cases, downflow applications with upflow regeneration and backwash were considered. The assumption was made that the influent selenium concentration would be about 1.9 mg Se/L.
Loading to the Alumina to the Adsorbers
The heat of wetting produced when activated alumina is mixed with water causes cementation of the alumina bed. To prevent cementation, the alumina should be loaded to the adsorbers as a slurry.
Backwashing the Fixed Bed Adsorbers
After loading to the columns, it is necessary to backwash the media to remove the fine alumina grains, and after regeneration it is necessary to wash spent NaOH and HCl solution from the alumina bed. If the fines are not removed, the headloss through the adsorbers will increase.
If no special mechanism is used to retain alumina during backwash, a significant amount of alumina may be lost. Given the experimental data described above, the choice of the alumina grain size depends on the mechanism the design engineer uses to prevent alumina losses during backwashing and regeneration. Generally, for 28 x 48 mesh alumina, a backwash flowrate of 11.5 gpm/sq ft was required to fluidize the alumina bed approximately 50%. For 30% expansion, a flow rate of 8 to 9 gpm/ sq ft was required. In either case, we recommend using a bed expansion of 30 %. Larger bed expansion values may be used but alumina losses will be higher. Smaller bed expansions may be used, but will take longer to backwash the column. While not tested during the present study, the use of air and water backwashing combinations are considered likely to provide advantages to backwashing and bed cleaning in full scale alumina filters by breaking up agglomerations of alumina and filtered solids.
Selenium and Silica Adsorption Capacity
As mentioned previously in this paper, the presence of silica in the leachate caused the selenium adsorption capacity to be much smaller than desirable because some of the adsorption sites were occupied by silica. About 68% of the selenium contained in the leachate could be removed by activated alumina. The remaining 32 % consisted of an unidentified selenium species. Silica was present in the leachate in high concentrations (average 24.0 mg Si/L) and also was adsorbed by the alumina. Silica was determined to be a constraint in the adsorption of selenium from the leachate because it decreased the selenium adsorption capacity.
The 68 % removal could be obtained without changing the pH of the leachate. This was possible because most of the selenium in the leachate was in the selenite (Se IV) form. However, the selenium removal increased to about 84% at pH values about 4. In addition, silica adsorption was very poor at pH < 4. Despite the apparent advantages of decreasing the pH of the leachate to values < 4, it may not be possible because at low pH values hydrogen cyanide is released from the mine leachate and safety becomes an issue.
The following rationale applies to the calculation of the selenium adsorption capacity for the activated alumina: it is considered that at the feasible operating pH, approximately 0.6 mg Se/L from an unidentified selenium species cannot be adsorbed by activated alumina. This approach assumes that since this 0.6 mg/L of selenium did not exist as selenite or selenate, and since it can not be readily removed by activated alumina, it would not be considered in effluent discharge requirements. Therefore, it is assumed that breakthrough of alumina would occur at 0.1 mg/L total removable selenium or 0.7 mg/L total selenium in the effluent. The value 0.1 mg/L was chosen because it is 10 times the drinking water standard for selenium, which seems to be the treated selenium concentration required by a number of the western states.
Figure 12 shows that breakthrough occurred at approximately 175 bed volumes when treating a leachate containing 1.9 mg/ L selenium. In other column tests, breakthrough varied from 140 to 180 bed volumes. Therefore, 175 bed volumes to a breakthrough of 0.7 ppm effluent selenium was assumed for design. It was observed, as shown in Figure 12, that selenium breakthrough (0.7 mg/L) normally occurred at silica effluent concentrations greater than 1.0 mg/L. Based on this parameter, the selenium adsorption capacity for the leachate was calculated as 5.6 g of Se/cu ft of alumina. The silica adsorption capacity was calculated as 103.0 g Si/ cu ft of alumina. Columns fed synthetic selenite solution were operated to about 900 bed volumes, and a selenium loading of approximately 24.4 g Se/cu ft of alumina was obtained.
Activated alumina suspensions are very alkaline with pH’s varying from 11.5 to 12. This fact influences the absorption of selenium when the alumina is first used. Backwashing the alumina reduces the pH somewhat. In a full scale operation, the alumina may be washed by the solution to be treated (leachate), and during initial application, the leachate may need to be recirculated to the influent for retreatment. The amount of recycle can be determined by measuring the effluent pH and selenium concentration at start-up. This practice is similar to that used in water filters to reduce the Impact of the first flush on the treated water quality.
Empty Bed Contact Time (EBCT)
The empty bed contact time is the average fluid detention in the empty bed and is defined as EBCT = V/Q; where Q is the volumetric flow rate and V is the total alumina bed volume including voids. The studies performed with the mine leachate indicated that there was no significant difference in selenium adsorption for EBCT’s ranging from 2.0 to 14.0 minutes. From an operational point of view, it would be desirable to process as much leachate as possible in a short period of time. However, for a given adsorber size, the head loss in the column must also be considered. Head loss data (Table 3) indicated that a low head loss ( < 1.0 foot) occurred at flow rates as high as 9.6 gpm/sq ft. Based on this, alumina loading rates ranging from 5 to 10 gpm/sq ft can be used. Because most of our column testing involved EBCT’s greater than 6 minutes, we decided to use an EBCT of 6 minutes for design. However, Yuan (1984) have shown that an EBCT of 1 minute was sufficient for selenium adsorption.
At an EBCT of 6 minutes and a loading rate of 7.5 gpm/sq ft, an alumina bed depth of 6 feet would be required. At 175 bed volumes between regenerations, the cycle time would be approximately 18 hours.
Alumina Regeneration
The regeneration of the spent alumina may be accomplished in the following steps:
- Drain excess water from the alumina bed.
- Add 0.25% to 0.5 % NaOH to saturate the bed (3 to 5 bed volumes (BV)).
- Let stand approximately 1 hr.
- Backwash the bed with effluent for 5 to 10 BV to remove NaOH and excess Se and Si.
- Rinse bed with 3 to 5 BV of 0.25% HCl.
- Return adsorber to service.
NaOH concentrations as low as 0.25% were sufficient to remove selenium and silica from the alumina. Higher NaOH concentrations resulted in large alumina losses by dissolution. Use of weaker NaOH solutions for regeneration would produce larger volumes of regenerant as compared with the use of higher regenerant concentration. Thus, the choice of the best NaOH concentration will be related to the spent regenerant disposal costs and alumina losses.
The alumina loss was calculated for various regeneration tests by measuring by ICP the aluminum concentration in the spent regenerant. To convert aluminum concentration into alumina loss, it was assumed that activated alumina is composed of 95 % aluminum oxide. Alumina losses ranging from 0.2 to 2% were found for the different regeneration flow rates. It is necessary to emphasize that the alumina loss calculated is that which was dissolved by NaOH. In order to calculate the total loss of alumina, it would also be necessary to account for the alumina lost as particulate during regeneration. Alumina losses as high as 10% have been reported for full-scale plants (Yuan , 1984 and Sorg, 1978).
The last step of the regeneration process involves rinsing the alumina with HCl. In our testing, three bed volumes of 0.25% HCl where used to recharge the alumina. This volume was not calculated, but was based on published recommendations. No tests were performed to optimize the acid recharge time because the results obtained were satisfactory.
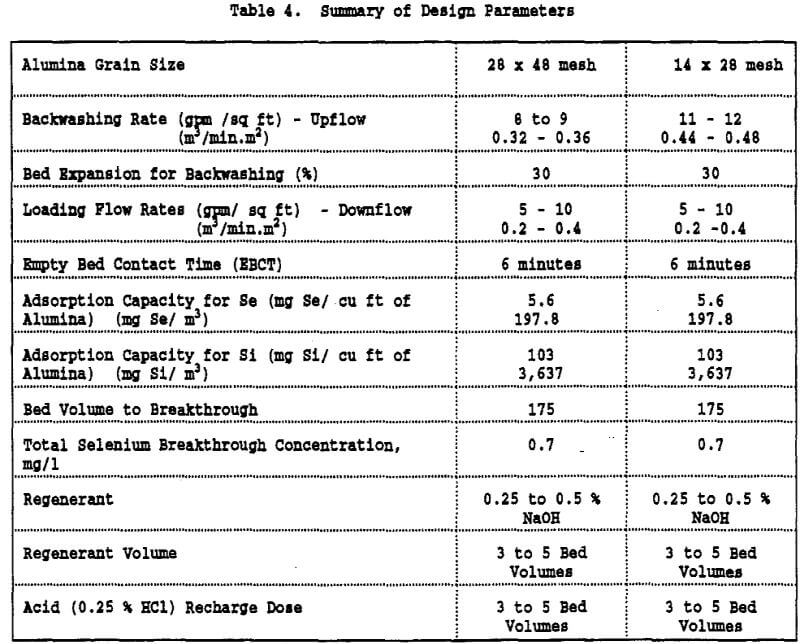
Regenerant Solution Disposal
The waste solutions produced upon the regeneration of spent activated alumina will contain mainly high levels of selenium, silica, and moderate levels of arsenic, which is also adsorbed by activated alumina. The first spent regenerant solution is a NaOH solution and has a high pH. The second regenerant solution is HCl and will be acidic. Mixing of these wastes will help to neutralize the solution if desired. These regenerant solutions must be disposed of properly according to federal and state regulations, and costs of disposal of the regenerant solution must be considered in a full scale design. X summary of the critical design parameters computed from our study is shown in Table 4.