Table of Contents
In 1947 the author became interested in the fundamental aspects of the desilverizing of lead by zinc, conducted some experimental work, and searched the technical literature for all available fundamental data. Since then a revival of interest in the subject in Europe resulted in the appearance of quite a number of papers. It became evident that it would be more profitable to collect together and examine thoroughly the results of various workers, than to attempt to duplicate the experimental determinations.
Historical Introduction
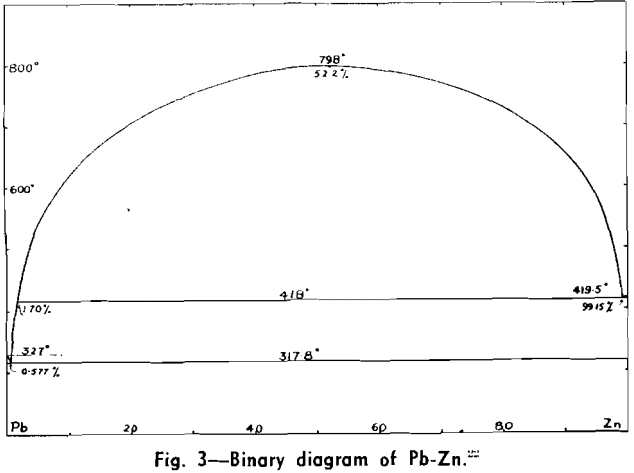
The correct theoretical interpretation of the phenomena occurring in the desilverizing process was not given for many years although various statements occur in the older text books, some partially correct, but many completely erroneous. The liquid miscibility gap which occurs in the binary Pb-Zn system, and hence also in the ternary system Ag-Pb-Zn, was usually considered to be bound up with the phenomena. The first determinations of its extent were made by Wright and Thompson in 1890.
Kremann and Hofmeier in 1911 were the first to show that the liquid miscibility gap has no bearing on the practical process, and later Hofman made this fact generally available to members of the German-reading world. The liquid miscibility gap of Bogitch in 1915, was much less accurately determined.
The practical applications by Williams of his theoretical findings should be too well known to warrant more than brief mention here. He used his cooling curves first for the accurate determination of the most economical zinc additions to be used for the two-stage batch desilverizing process; his liquid miscibility gap and tie lines for the development of
the crust enrichment process; and finally, his knowledge of the whole system for his now famous one-stage continuous desilverizing process which he termed the “conjugate solution process.”
In 1950 Johannsen and Lange-Eicholz re-examined equilibrium relations in the field where Williams cooling curves were determined, that is, between the liquid miscibility gap boundary and the eutectic trough in the lead corner. Their experimental method was most ingenious: holding lead at constant temperature in an Ag-Zn crucible of known composition and sampling the lead solution after it was presumed equilibrium had been established.
The position of the eutectic trough found by Johannsen and Lange-Eicholz is stated to confirm Jollivet’s results although their drawings show it in a rather different position. Finally, Johannsen and Lange-Eicholz found a ternary eutectic point near the binary Pb-Zn eutectic which, however, is geometrically impossible, since this point lies outside the triangle joining the limits of composition of the ε + η region with the lead corner.
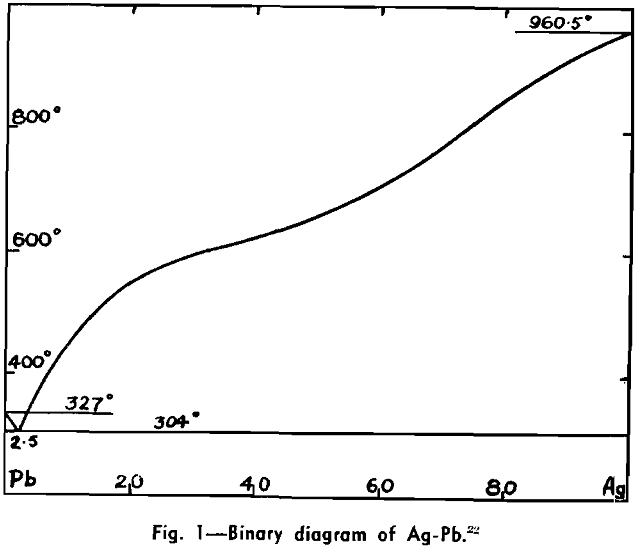
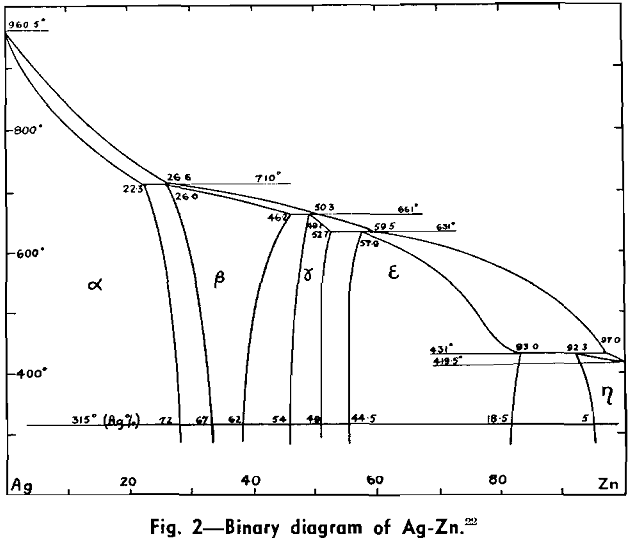
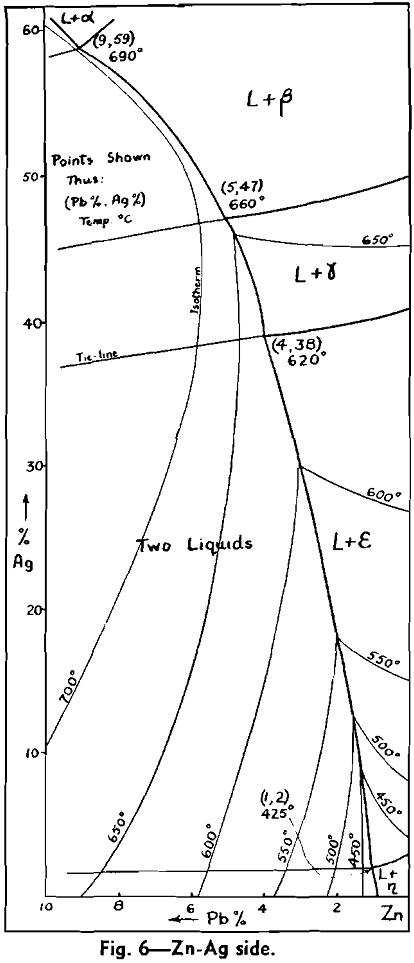
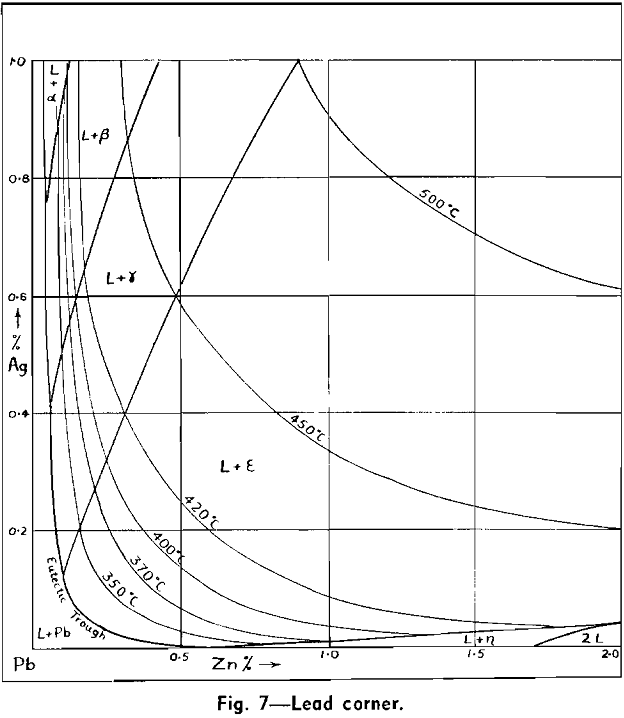
The Ag-Pb-Zn Phase Diagram
The representation of any ternary diagram as complicated as the Ag-Pb-Zn system is difficult. If all solidus and liquidus boundaries are shown, the diagram becomes almost unintelligible. It is perhaps due mainly to the difficulty experienced in understanding other authors representations and descriptions that so many publications relating to this system reveal apparent neglect or misinterpretation of what has gone before.
The present paper attempts to deal with the difficulty in the following way: Scale drawings are presented, showing the entire diagram on an equilateral base with only liquidus phase boundaries and isotherms marked; the lead side and the zinc side of the liquid miscibility gap on rectangular co-ordinates; and two greatly magnified sections of the lead corner on rectangular co-ordinates. Then schematic representations, not to scale, are used to illustrate the course of a number of desilverizing procedures.
The central liquid miscibility gap, or conjugate solution region, is crossed by a number of tie lines to show the compositions of solutions mutually in equilibrium. The tie lines drawn are those connecting the peritectic invariant points on either side of the miscibility gap.
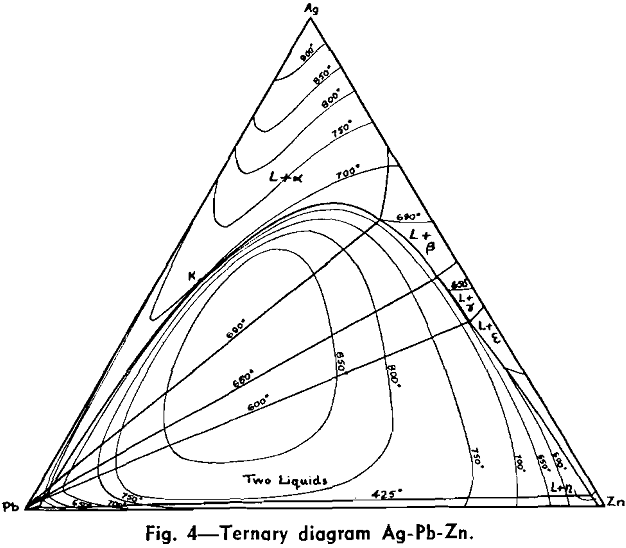
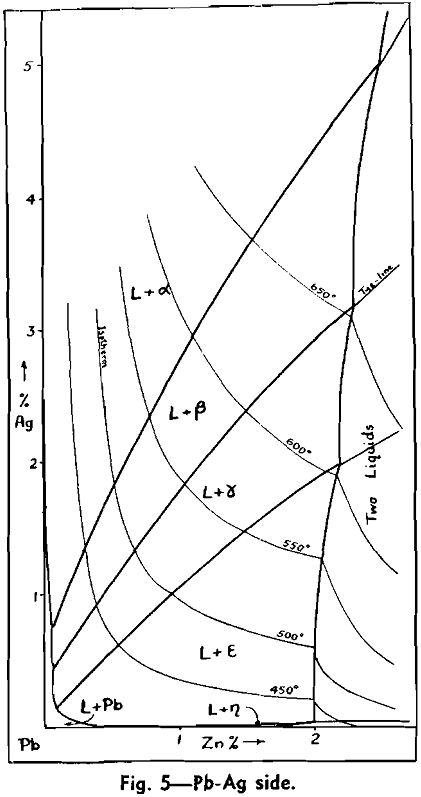
No temperatures have been shown on the eutectic trough, since the only information available is that it is practically an isotherm at 315°. It is theoretically necessary, however, that the trough has a temperature maximum in the region sloping downward both past the ε — η peritectic transformation point to the Pb-Zn eutectic, and past the ε — γ, γ — β, and β — α peritectic transformation points to the Ag-Pb eutectic. This fact has not been recognized in previous publications of this diagram, but arises from consideration of a few geometrical necessities of the graphic representation of phase equilibria, in conjunction with the cooling curve data.
Practical Desilverizing Processes
It is possible to desilverize lead to very low limits using any number of stages, from one up, of zinc addition. The greater the number of stages, the less is the total zinc consumption in production of Ag-Zn alloy or crust (provided that crusts are returned from each stage to the preceding one) and the lower the temperature required for stirring in each stage. But the time requirement and the zinc loss by oxidation are both greater. The zinc consumption for saturation of the desilverized lead is, of course, independent of the number of stages.
The practical difficulty which precludes the use of only one stage in most cases is that of dissolving all the zinc required at a high temperature without severe loss by atmospheric oxidation. For example, a lead containing 0.2 pct Ag (65 oz per long ton) requires about 2 pct Zn to be stirred in at a temperature exceeding 450°C, in order that the lead when cooled to its freezing point shall contain about 0.0005 pct Ag (0.16 oz per long ton).
Batch Processes, Using Zinc in Two Stages: The possible varieties of this procedure have been discussed so thoroughly by Williams that it is unnecessary to repeat here at length the advantages and disadvantages of each variation. The three main ones are illustrated schematically in Fig. 10, and for convenience are designated “Pirie”, “American,” or “German.” The Pirie method has been reported frequently, the German and American methods have been discussed.
If the bullion varies in silver content from batch to batch, the zinc additions required for a number of procedures are listed in the very complete zincing tables of Williams.
If less than 0.0005 pct Ag in desilverized lead is desired, then it is necessary that the cooling curve enters the phase region L + η. At least some of the final crust will have an Ag/Zn ratio of less than 5/95 = 0.0526, so that the rich crust produced will also have a lower Ag/Zn ratio than the previous case, and the overall zinc consumption will be greater.
Isothermal Low Temperature Batch Process: This process is fully described in the German literature but will be described here briefly because, to the author’s knowledge, no English reference has yet been published.
Fig. 12a shows, on the 330°C isothermal phase diagram, the course followed by bullion composition during the process. (The fact that the bullion temperature rises from 330°C to 340°C and then falls to 320°C can be ignored for the sake of simplicity.) The original bullion is placed in a kettle in which the second crust of the previous charge remains. One third of the total zinc addition is made while the kettle is stirred, the bullion temperature being about 330°C. T
Before reading ref. 19, the present author had, in laboratory tests, desilverized 5000 gram charges of bullion from 60 oz per long ton silver to a fraction of an ounce per ton, by stirring in solid zinc at 350°C for 1 hr, and cooling to the lead freezing point.
Williams Continuous Desilverizing Process: It has already been pointed out that a batch of bullion can be completely desilverized with one zincing at a high temperature followed by cooling to nearly freezing.
In the Williams kettle, lead containing silver is introduced at the top at high temperature (about 600° to 650°C) where a layer of molten zinc is maintained. At this temperature about 10 pct Zn can be absorbed by lead. The lead flows downward through the kettle, of which all but the top section is cooled by air draught. (The top section is fired to keep the zinc-rich layer molten.) As the temperature of the lead falls, the saturation limits of zinc and silver progressively fall, so that solid ε-phase Ag-Zn is continuously rejected. The cooling of the kettle is so arranged that at the bottom lead is very close to its freezing point, and thus its composition is at a point very close to the eutectic trough.
Port Pirie practice is to dip off the silver alloy layer when its appearance indicates that it is about to solidify. The desilverized lead at that stage still contains less than 0.0003 pct Ag, and in fact it has not been demonstrated that the silver content of desilverized lead rises throughout a cycle.
When the silver alloy has been dipped off, fresh zinc is added to the top of the kettle where it melts, and a new cycle commences, the alloy layer moving in composition from pure zinc to A1 and then gradually to A’1. The lead flow is not stopped while skimming the alloy or adding fresh zinc so that the lead flow is continuous, whereas removal of the alloy is discontinuous. Once a week the lead flow is stopped, the kettle sides heated to free adhering crusts which have not floated, and the kettle bottom cooled to nearly freezing before the lead flow is recommenced. The stoppage is normally for 8 hr.
Degolding by The Parkes Process
At one time it was general practice at lead refineries using the Parkes process to produce a so-called gold skim before desilverizing proper. Practically all the gold and copper, together with a fraction of the silver, are removed by adding a relatively small amount (0.3 pct) of zinc to the bullion.
The explanation invariably given for this phenomenon is that “zinc has a greater affinity for gold and copper than for silver.” It is difficult to improve upon this explanation, unless the more sophisticated expression is preferred: that the free energies of formation of Zn-Cu and Zn-Au phases are greater than those of Zn-Ag phases. Figures are quoted for phases of unspecified composition by Henglein and Nowotny, namely, —5.2, —2.8, and —2.2 kcal for Au-Zn, Cu-Zn, and Ag-Zn, respectively. The differences are great enough to ensure the removal of practically all the gold and copper with as little as 13 pct of the silver in practice. The gold to silver ratio in the gold skim was increased by recycling crusts from treated batches to succeeding batches. In the case cited the Bunker Hill smelter adopted the Port Pirie practice of recycling the gold crusts through five kettles.
The Cu-Au crusts are not recycled through No. 1 retort, but are retorted separately and enriched by recycling through this second retort and cooling kettle to any desired extent. The final very high grade Au-Cu crusts, which are a very small fraction of the original retort bullion quantity, are removed, retorted if warranted, and then cupelled and parted.
The economic evaluation of such a treatment depends upon the balance between power and labor saved in the parting plant plus lead returned directly to the refinery, as against slightly increased retorting costs, the labor cost of the degolding process, and zinc lost by oxidation when recycling crusts, although there could be a net zinc gain if less zinc were lost in cupellation.

