In the crucible-method of assaying ores for silver a certain amount of litharge is essential to supply sufficient lead to collect the precious metals. The object of this paper is to point out that the use of a large excess of litharge in the assay of some ores will give results for silver that are uneven as well as low. So far as I know, however, an excess of litharge does not affect the results obtained in the crucible-assay of ores for gold.
The main reasons for using, in the crucible-assay, much more litharge than is required to give the necessary lead button are:
- its action as a flux;
- its action as a desulphurizer; and
- its action as an oxidizer, especially on metals like copper and nickel, whereby they are forced into the slag as oxides and thus prevented from passing into the lead button.
Hence, by the use of much litharge in the crucible-assay more ore can often be taken than in the scorification-process, and a lead button obtained which can possibly be cupelled at once or after a single scorification. The method is specially advantageous with an ore carrying much copper or similar impurity and poor in silver, when the assayer does not wish to resort to a wet-analysis for the determination of the silver.
It is well known that some ores give better results when assayed by the scorification-method, while others give better results by the crucible-method.
For several years I have noticed that, when much litharge was used with certain sulphide and arsenical ores, the results were considerably lower than when the scorification-method was used.
This fact was more forcibly brought out in connection with certain work carried on this year by Messrs. H. A. Frame and F. C. Jaccard, students at the Massachusetts Institute of Technology, to ascertain the best method of assaying the rich arsenical nickel- and cobalt-ores from Ontario, Canada.
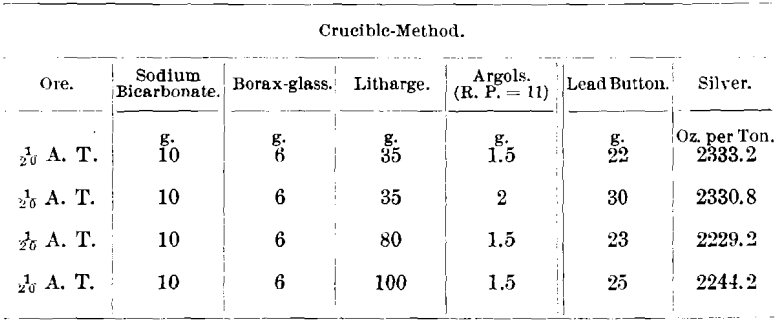
The following are some results obtained: Ore No. 2,687-2 had a reducing-power of 4.2 and contained Ni, 12.92; Co, 10.92; and As, 46 per cent. The minerals noticed in the ore were niccolite, smaltite, erythrite, cobaltite, and arsenopyrite.
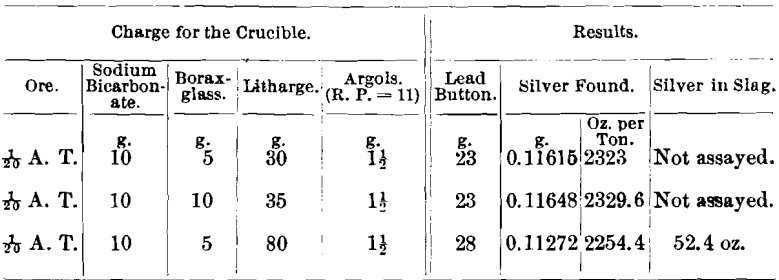
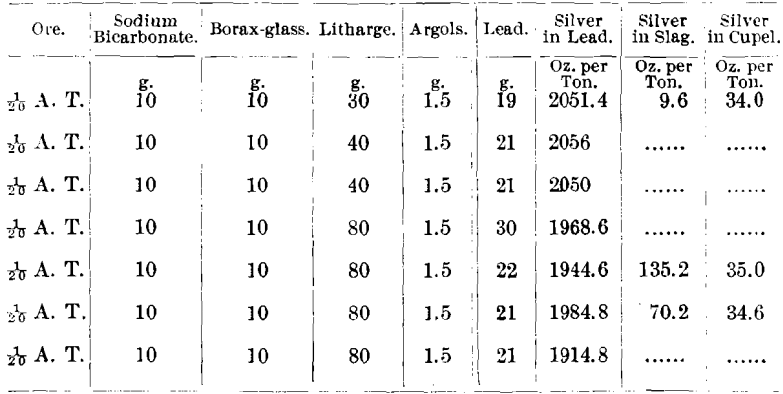
Ore No. 2,687-6 consisted chiefly of smaltite, erythrite, niccolite, and arsenopyrite; R. P. = 4.06; Ni, 3.94; Co, 11.25; and As, 59.7 per cent.
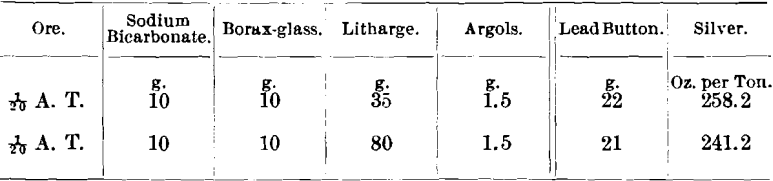
The same ore assayed after amalgamation:
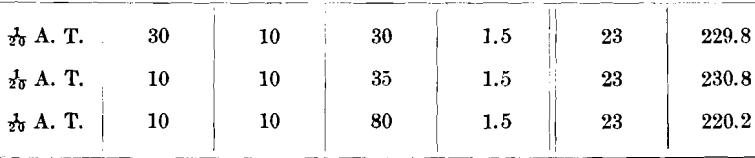
Subsequent to the above tests I made further investigations on the ores tested as well as on other ores and obtained the following results: Ore No. 2,687-2; R. P. =4.2; Ni, 12.92; Co, 10.92; and As, 46 per cent.
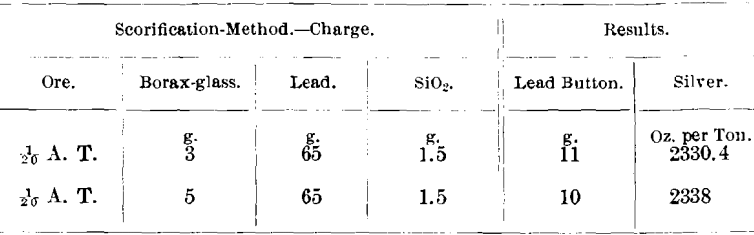
Ore A. Chiefly smaltite and niccolite with free silver, containing Ni, 5.06, and Co, 9.12 per cent.
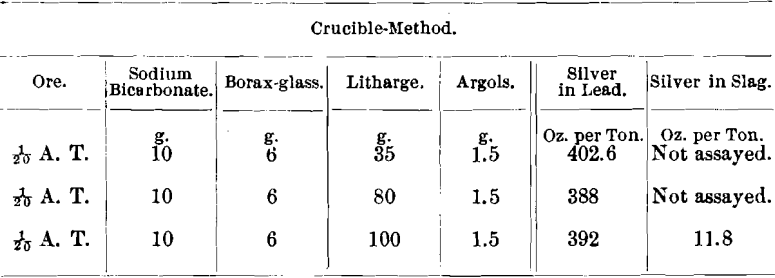
Ore No. 2,703-1 consisted of smaltite, native bismuth and native silver in calcite; R. P. = 5; Ni, 0.3 ; Co, 8; and As, 55 per cent. The scorification-assay gave 404.8 oz. and the combination wet-and-dry analysis gave 403.7 oz. of silver per ton.
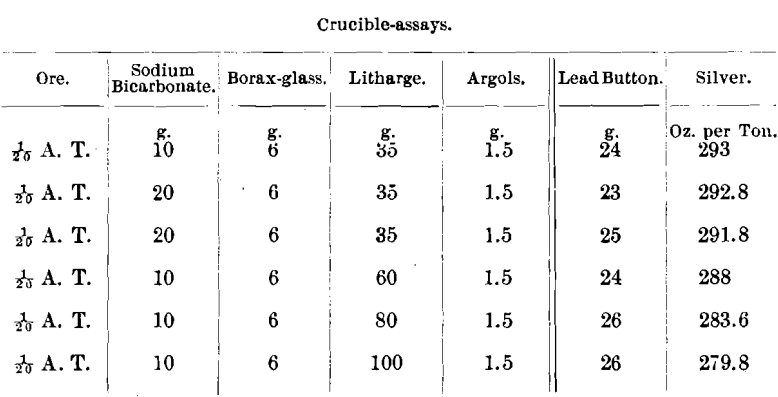
The same ore after amalgamation gave, by the scorification- assay, 292 and 291.2 oz., while by the combination wet-and-dry analysis the result was 292.2 oz. of silver assayed per ton.
I have a number of other examples, but these should be sufficient to illustrate the fact to which I wish to call attention. The ores used carried practically no gold.
The same lot of litharge was used in all the fusions, and the conditions under which the fusions were conducted were as nearly identical as possible. Heavy crystals of litharge were found on all the cupels.
Only 1/20 A. T. of ore was used in the assays, because Messrs. Frame and Jaccard found that in case of ore No. 2,687-2, carrying 12.92 per cent, of nickel, if 5 g. of ore were taken and 35 g. of litharge were used, the resulting lead button would not cupel. If 5 g. of ore were taken and the litharge increased to 80 g., in order to slag the nickel, the button would cupel but the silver-results were low.
Some ores from the Cobalt district carry so large a percentage of nickel that 1/20 A. T. of ore is the limit that can be used in either scorification- or crucible work, and low litharge is inapplicable in the latter method. The following ore serves as an illustration: Ore No. 2,703-2, niccolite (NiAs); R. P. = 5.3; Ni, 38.01; Co, 1.19; and As, 53.31 per cent.
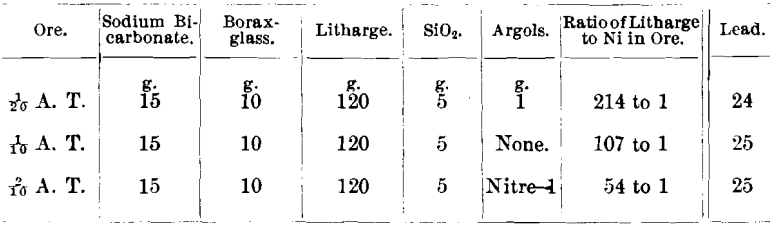
The lead buttons from 1/10 and 2/10 A. T. of ore would not cupel, both cupels being covered with a thick film of green nickel oxide (NiO).
The lead button from 1/20 A. T. of ore would just cupel, leaving the cupel stained green.
One of the advantages of the crucible-method is that it enables the assayer to use a larger amount of ore than in the scorification-method, but from the foregoing data there seems to be no advantage in this method over scorification on ores carrying much more than 10 per cent, of nickel. When, however, the ores, either siliceous or calcareous, are poor in silver and carry only a small percentage of nickel or similar impurity, the crucible-method, with low litharge, can be used to advantage.
Cobalt is much more readily slagged than nickel, especially in the presence of alkali and silica, and does not so readily pass into the lead button, therefore ores quite rich in cobalt can be assayed by the crucible-method, a considerable amount of ore be used and the litharge kept low.
From 80 to 90 per cent, of the cobalt, if a reasonable amount of ore is used, will pass into the slag in either scorification- or crucible-work.
It is a question with me as to the cause, in certain cases, of the uneven and low results in silver when high litharge is used in the crucible-assay, but apparently the silver passes in some way into the slag. At first I thought that, in the examples given, either the arsenic, the nickel or the combination of both was the cause, but this was not true of all ores. Some ores carrying considerable nickel, cobalt and arsenic gave good results as well as an ore carrying Co, 0.67; Ni, 0.44; and As, 0.6 per cent, in a gangue consisting of silica and calcite.
The question of high or low temperature does not seem to influence the slagging of the silver unless this takes place at some particular period of the fusion. Owing to this uncertainty as to the action of the litharge, I prefer the scorification-method for ores from the Cobalt district which carry much nickel, using 1/20 or 1/10 A. T. of ore, from 3 to 8 g. of borax-glass, high lead (65 g. or more), with some silica, and a medium high temperature. If obliged to use the crucible-method, I keep the litharge low and take such an amount of ore that both high litharge and nitre are avoided.
If all ores from this district are ground very fine, thus removing the greater part of the silver pellets, it is surprising how uniform are the results obtained by the scorification-method— something that I have not found by the crucible-method nor in some ores when using 0.5 A. T. for the combination wet- and-dry analysis.
It is hoped that this paper will bring out the experiences of some other assayers.
