Table of Contents
The production of pre-reduced iron ore pellets involves the expenditure of considerable capital and energy. Both prereduced and oxide pellets can be produced in similar equipment by using reducing and oxidizing processes, respectively. However, the throughput for a 5-m-ID by 50-m-long kiln with a preheating grate is 425,000 metric tons per year of ore, according to Janke and Garbe. This is equivalent to about 310,000 metric tons per year of 95-percent-metallized product. A kiln of this size equipped with a preheating grate is capable of producing over 1 million tons of hematite pellets annually. An appraisal of induration under oxidizing and reducing conditions indicates the annual capacity of a metallizing plant (operating with similar equipment) would be about one-third that of an oxide pellet plant. Therefore, heat losses to the surroundings will be substantially higher for prereduced pellets.
Also contributing to higher energy consumption are the heat requirements for production of strongly reducing conditions and the endothermic reduction process itself. The process results in only partial combustion of the fuel or reductant by air, and a portion of the carbon monoxide or hydrogen must be utilized to reduce the iron oxides to metal. Because an excess of carbon monoxide or hydrogen must be maintained to insure metallization, a portion of fuel values exits from the reduction vessel unburned. The end result is a consumption of approximately 14 million Btu per ton of metallized ore or pellets.
Various schemes have been devised to utilize both the sensible heat and the calorific power of reactor off gases to preheat the incoming feed, and thereby lower the overall heat consumption. Serbent estimated the heat consumption could be decreased from 14 million to 12 million by utilizing the heat potential of the off gases from a reduction kiln to dry and preheat the incoming ore or pellets. However, the incoming feed was either lump ore or indurated hematite pellets and no statements were made concerning the effects of preheating upon the final product.
Since most low-silica iron concentrates are finely ground during beneficiation, some form of agglomeration is necessary prior to metallization. One exception is fluid-bed reactors, where agglomeration is accomplished after metallization. Existing commercial processes carry out the agglomeration independently of the metallizing reactor. However, several processes employ, or can employ, unfired iron ore pellets directly as feed to the metallizing reactor. In these procedures induration is effected during metallization by the sintering of metallic iron grains. The final product is a strong, porous, metallized pellet which is essentially free of cracks.
Each style of operation has its advantages and disadvantages. Processes utilizing lump ore or fired pellets as feed generally do not suffer from sticking problems or accretion in the reactor; however, the reduction tends to crack and break much of the feed into chips. The end product then may need a reagglomeration (as by briquetting) to make it physically acceptable. On the other hand, the reduction-induration of green balls suffers from degradation of the unfired agglomerates; this may result in accretion on the reactor walls, although the final product would probably be somewhat superior in size distribution and physical strength.
From the foregoing it would appear advantageous to develop a compromise between processes utilizing completely indurated agglomerates for feed and those utilizing green, unfired pellets. If in so doing the product quality can be improved while decreasing costs, prereduced agglomerates will become economically more competitive. Preheating, when carried out to the optimum level, would indurate the unfired agglomerates sufficiently to prevent sticking and accretion, but not so completely as to result in excessive cracking, chipping, and spalling during the metallization process.
To determine the technical feasibility of subjecting green balls to a drying-preheating cycle prior to metallization, a laboratory investigation of the proposed process was carried out. The physical properties of the pre-heated and metallized balls were correlated with the preheat temperature. Of particular interest were compressive strengths of the preheated balls prior to reduction and the resulting effect of preheating on the final cohesiveness and compressive strength of the metallized pellets.
Assuming that excess reductants such as carbon monoxide, hydrogen, or possibly unburned fuel gases exist in the off gases, it would be necessary to burn these gases to realize the maximum thermal potential of the system. Depending upon the quantity of secondary air added, the preheating conditions could vary from reducing to oxidizing. However, the most likely conditions would be somewhere between neutral and oxidizing to insure combustion of all fuel values. Because duplication of possible furnace atmospheres is at best difficult, air was chosen as the sole oxidizing atmosphere for preheating experiments.
Raw Materials
Three magnetite concentrates (I-III), a fine hematite screen concentrate (IV), and a hematite flotation concentrate (V) were used as feed materials for this investigation (table 1). The magnetites were selected because they represented high, average, and low-silica concentrates (I-III, respectively). Structure of concentrates II and III was approximately the same, each being essentially 90 percent minus 325 mesh and having a surface area of about 2,500 cm²/g (table 1) as determined with the Blaine air permeability apparatus. Concentrate I, 80 percent minus 325 mesh, was somewhat coarser, having a Blaine surface of 1,800 cm²/g.
Concentrate IV was a fine-ground hematite screen concentrate used for comparison purposes to determine if a natural hematite would exhibit the same properties as an oxidized magnetite.
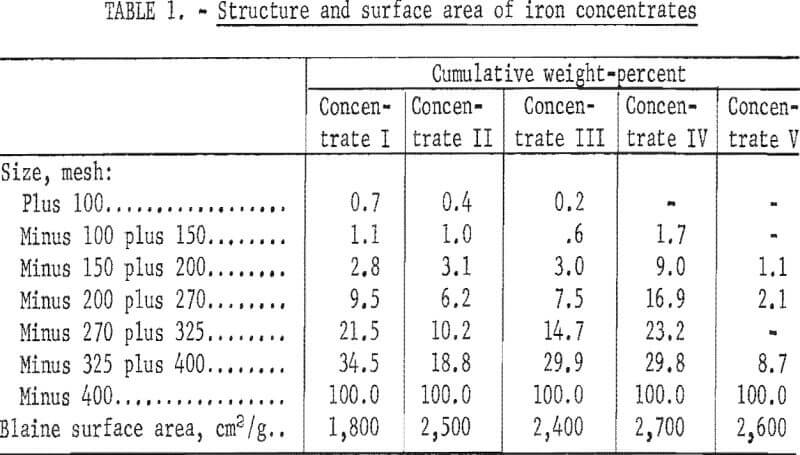
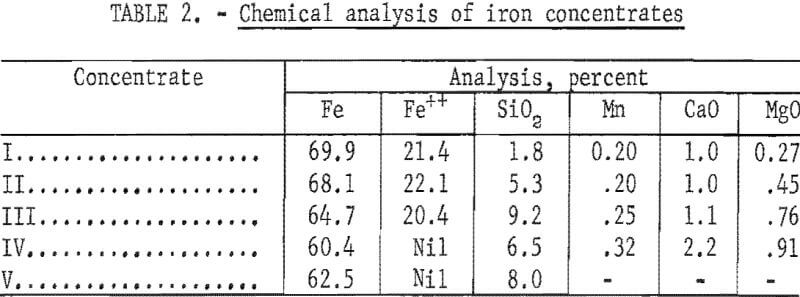
Concentrate V was a hematite flotation concentrate produced by the Bureau of Mines “Selective Flocculation” process. Chemically the concentrates analyzed from 62 to 6 7 percent iron and from 3 to 9 percent silica (table 2). A North Dakota lignite charred at 450° C was used as a reductant.
Procedure and Equipment
Pelletizing
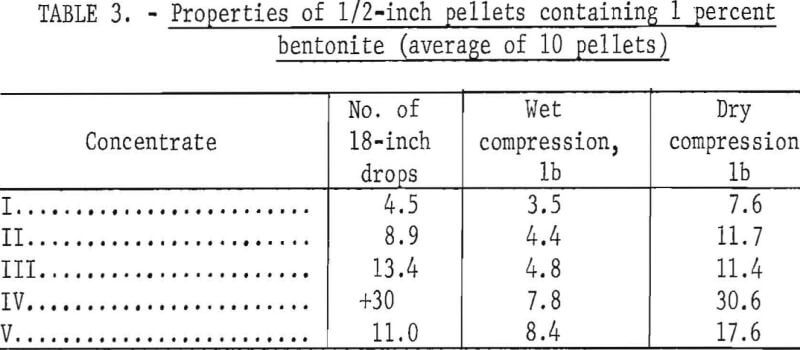
The ½-inch pellets used for this study were prepared by a standard laboratory balling procedure. An unlined stainless steel drum, 16 inches in diameter by 5 inches wide and rotating at 52 rpm, was used initially to make seed pellets and then to build finished pellets by the addition of moist concentrate plus water from a sprayer. Finished pellets were sized to minus 9/16 plus ½ inch prior to testing. Individual spheres were then evaluated for 18-inch wet drop number, wet compression strength, and dry compression strength. The bentonite binder addition was maintained at 1 percent of the dry concentrate weight.
Table 3 presents properties of pellets derived from the five raw materials. With regard to the magnetites, the strongest pellets were made from concentrates highest in silica. The concentrate IV pellets (hematite) were exceptionally strong due to the presence of earthy iron oxides which supplemented the bonding action of bentonite, while concentrate V, which was deslimed during flotation, produced somewhat weaker pellets.
Experimental Work
The preheating-reduction process was carried out as follows:
- Two hundred g of oven-dried pellets were charged to the drum under nitrogen (flow rate of nitrogen 3 liters per minute).
- The drum was placed in an electric muffle preheated to the desired temperature.
- When the furnace had recovered preheat temperature, the atmosphere was changed to air.
- At the conclusion of the preheat cycle, five pellets were removed for testing and the furnace was heated to 1,000° C.
- At 1,000° C the air was switched off, the lignite char was added, and reduction was allowed to proceed for 30 or 60 minutes.
- After completion of reduction, the drum was removed from the furnace and allowed to cool.
Step 5 involved charging of 75 g of char to the reactor. This was readily accomplished by ramming a paper tube containing the char into the reactor. No supplementary gaseous atmosphere was necessary, as the lignite provided a continuous emission of carbon monoxide.
Three criteria were considered in judging pellet quality. (1) Compression strength values of preheated pellets were used to estimate the durability of the product during subsequent processing; (2) after reduction, compression strengths of the metallized spheres indicated relative completion of the induration process; and (3) the tendency of the pellets to crack and spall was noted. Cracking was not considered serious as long as an adequate compression strength was maintained and no significant spalling occurred.

Equipment
Preheating and reduction were carried out in a 4-inch-ID by 4-inch-long type 316 stainless steel rotary drum. The drum was supported by a single 1-inch stainless pipe and closed with a threaded cap (fig. 1). Atmosphere was provided by passing the desired gas through a central ¼-inch stainless tube which extended into the drum through the hollow mounting shaft. Exiting gases flowed back through the shaft and were carried into the exhaust system. The drum was externally heated by inserting it into an electric muffle and sealing the furnace opening with insulating brick. Gases were metered to the drum by commercial flowmeters. This system made it possible to carry out the entire process in one vessel, making it unnecessary to transfer the pellets from one reactor to another. Samples were removed during operation by inserting a scoop through the mounting shaft and withdrawing five pellets.
Results
Oxidation of Magnetite
The heat released by magnetite oxidation constitutes a major energy benefit to the firing process during production of oxide pellets and promotes rapid pellet induration. Approximately 350,000 Btu/ton of pellets are released as a result of the exothermic reaction
2Fe3O4 + ½ O2 → 3Fe2O3.
Although this heat is not available in production of prereduced pellets, it may nevertheless be advantageous to oxidize magnetite to hematite while preheating. Several investigators have found the reduction to metal is significantly faster for hematite. If this phenomenon is also true of pelletized iron oxides, the capacity of reduction vessels may be increased by preheating magnetite balls under oxidizing conditions.
The following experimental procedure was designed to simulate a reduction system in which green balls would be dried and preheated on a grate, then transferred to the kiln. Solid reductant would also be fed to the kiln to accomplish the reduction. With this arrangement, the gases exiting the kiln would not only be hot but could have a substantial fuel value from the CO content. Optimum fuel utilization would then be attained by burning the kiln gases and using the fully oxidized hot gases for preheating.
Accordingly, pellets prepared from the five concentrates were preheated in air for 30 and 60 minutes at 500°, 700°, 900°, and 1,000° C. As would be expected, raising the temperature accelerated the oxidation rate of the magnetites (fig. 2). However, doubling the duration of the preheat from 30 to 60 minutes did not significantly affect the extent of magnetite oxidation, and the ferrous iron content was only slightly lower than the 30-minute results (fig. 3). Apparently at any given temperature the first portion of the oxidation is quite rapid, but then slows considerably. In each case, the oxidation curves are nearly parallel and ascend steadily with temperature until virtually all the magnetite is converted to hematite at 1,000° C. These results, coupled with petrographic observation of partially oxidized magnetite grains, indicate hematite forms readily along certain planes in the magnetite (fig. 4). The subsequent oxidation must then proceed by growth of the hematite
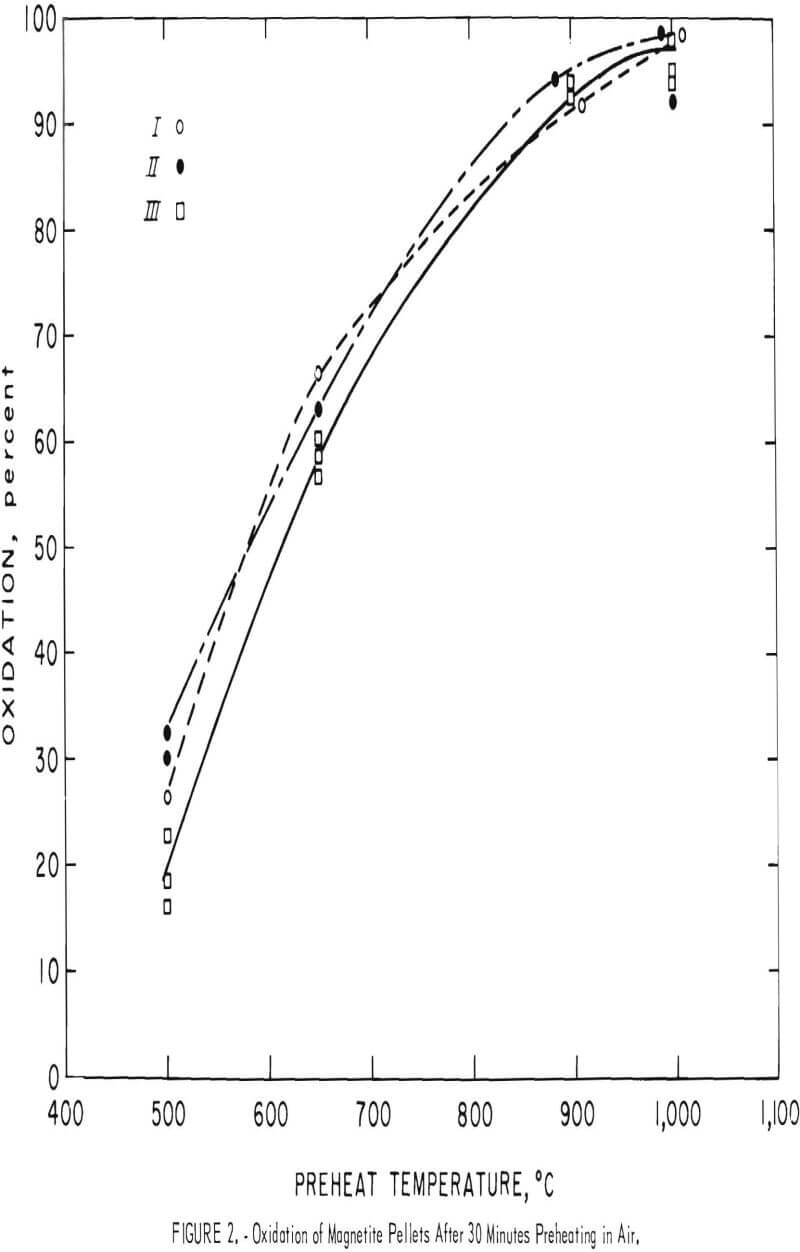
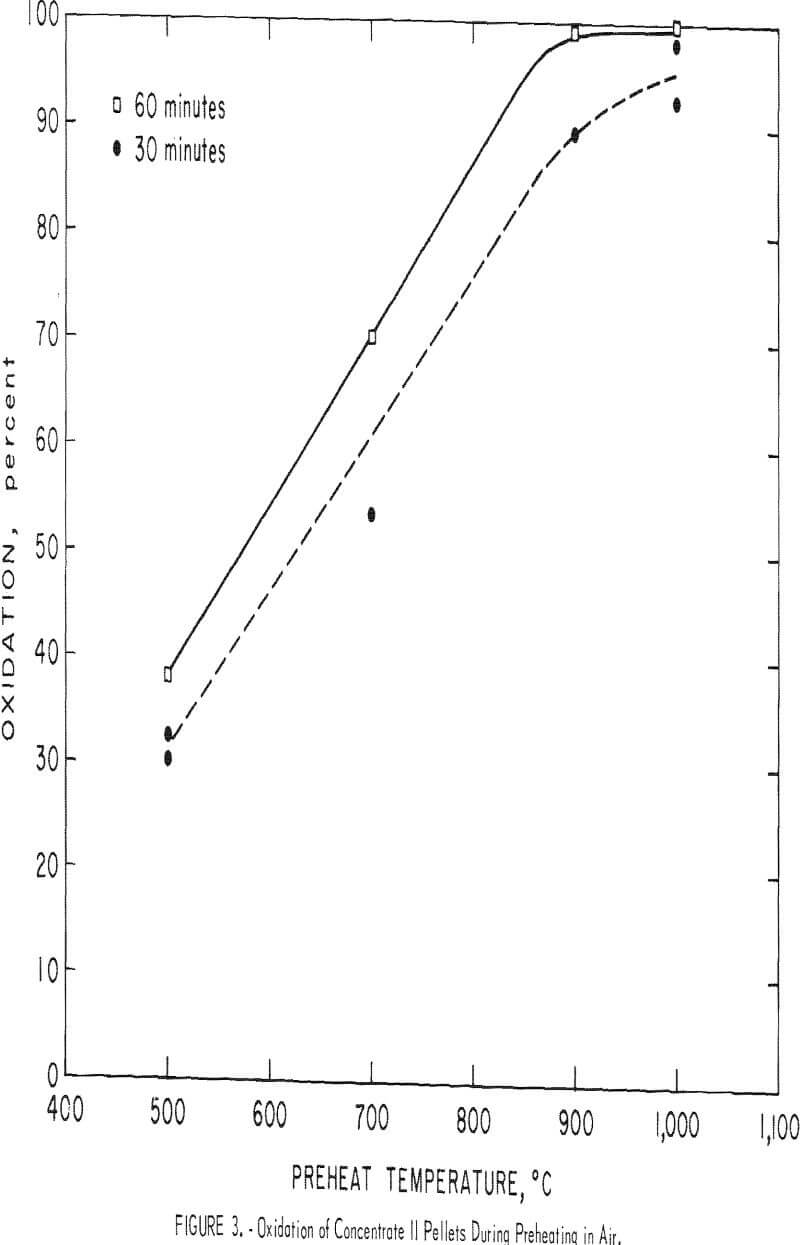

grains. However, increasing the temperature increases the extent of oxidation, until at 900° C oxidation is over 90 percent complete in 30 minutes.
Sintering
The oxidation of the magnetite did not alter the macrostructure of the oxide pellets and no cracking occurred at any of the selected preheat temperatures. However, each concentrate begins to sinter and undergo grain growth at elevated temperatures. The temperature at which sintering occurs at a detectable rate is significantly influenced by the mineral and chemical composition of the concentrate. Although each concentrate consists primarily of magnetite and silica, trace components can have significant influence on the sintering properties. For example, addition of 1.0 percent bentonite as a pellet binder has an effect of promoting the sintering rate and decreasing the minimum sintering temperature.
Compression testing was used to determine the degree of sintering occurring during the preheat cycle. Observation of polished sections was a less sensitive technique and did not reveal bond formation at 500° and 700° C, although the compressive strength had increased, indicating initial bond formation.
Effect of Preheating Upon Metallization
The indurating effect of preheating has a negative influence on the metallization rate of the pellets. This was most evident for concentrate IV (hematite), where effects from cracking and spalling were essentially eliminated (fig. 5). The metallization level decreases steadily from 88 percent
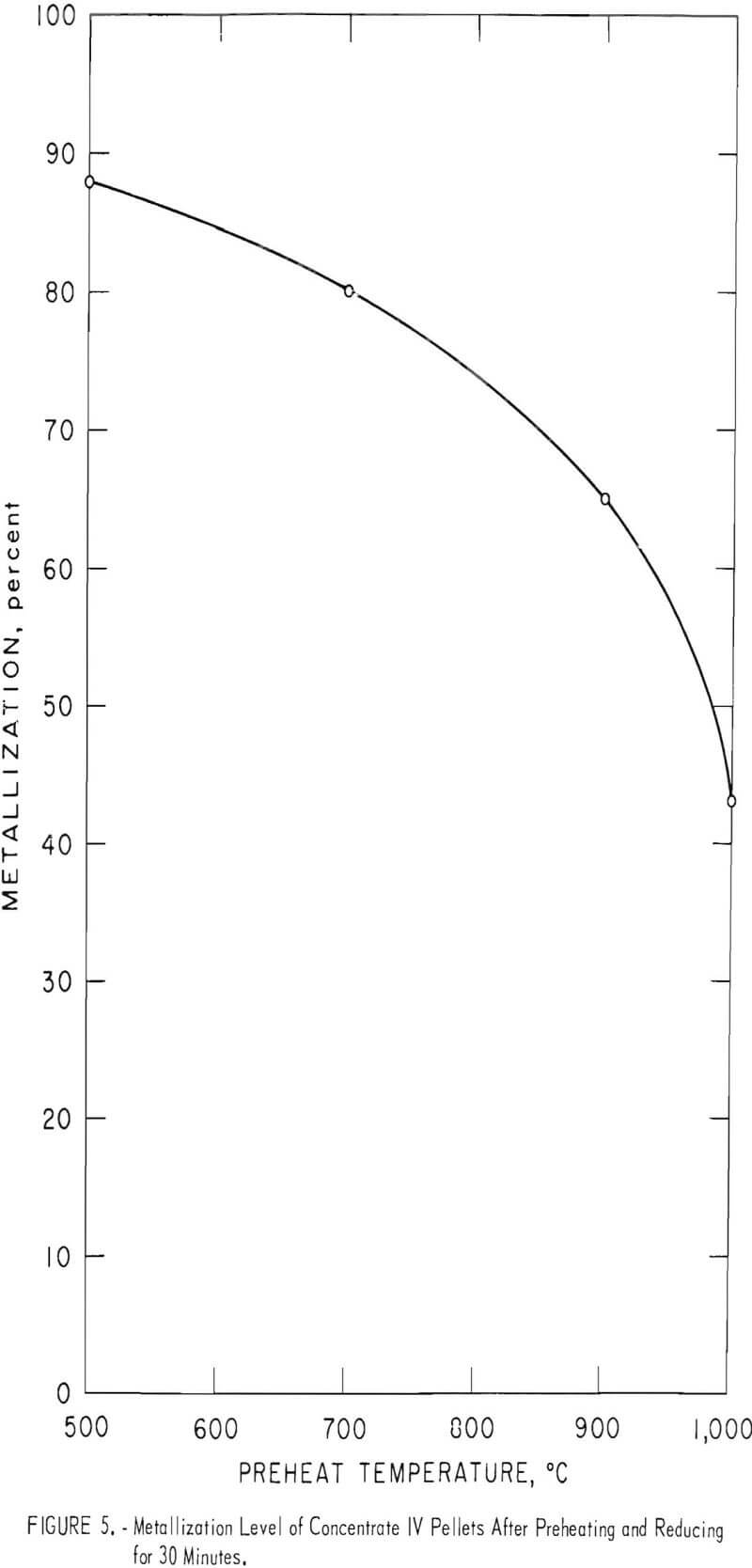
at a 500° C preheat to 43 percent at a 1,000° C preheat. It is evident that additional retention time or a higher metallization temperature would be required to improve the metallization level at the higher preheat temperatures. In fact, increasing the reduction temperature to 1,100° C produced 94-percent-metallized pellets at the 700° C preheat level, while increasing retention time from 30 to 60 minutes resulted in 90-percent-metallized pellets at the 900° C preheat level.
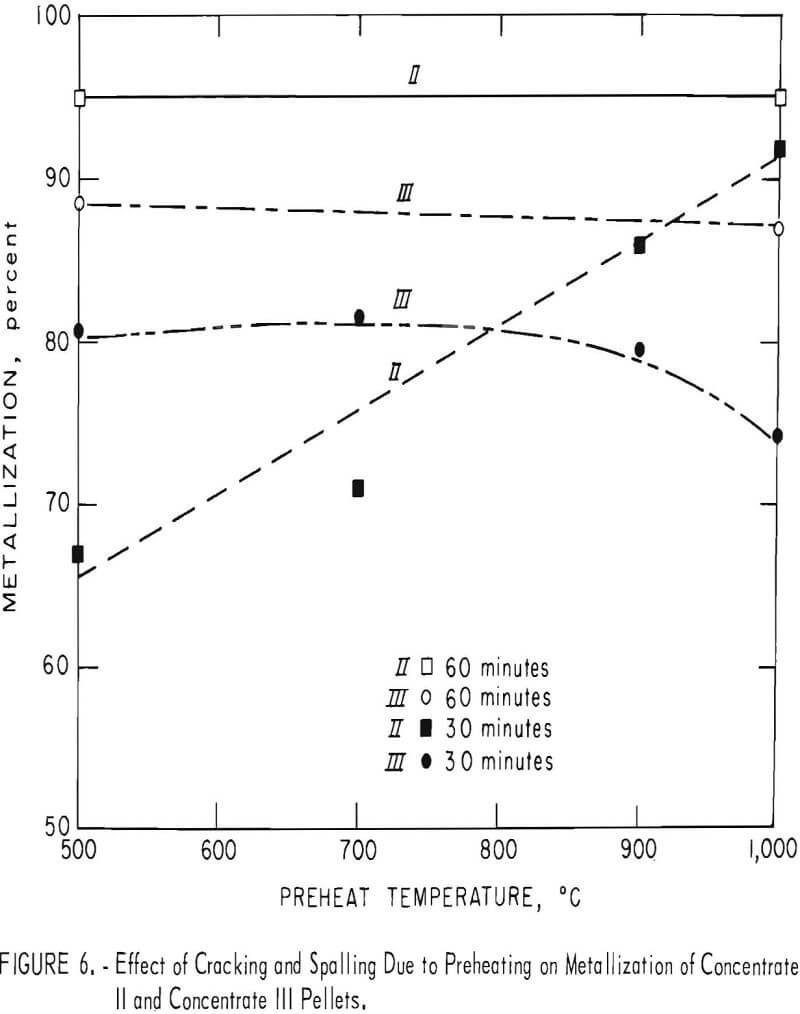
Concentrate II, a magnetite, demonstrated on the other hand a steady rise in metallization with increasing preheat temperatures from a 67-percent metallization at 500° C to a 92-percent metallization at 1,000° C (fig. 6, 30-minute line). However, cracking and spalling and not oxidation were responsible for most of the change, because concentrate III, also a magnetite, did not demonstrate either the severe cracking and spalling or the accompanying rise in metallization levels. Referring again to figure 6, to the 60-minute line, it can be seen that the metallization level has risen to the 95-percent level when the reduction time was increased to 60 minutes.
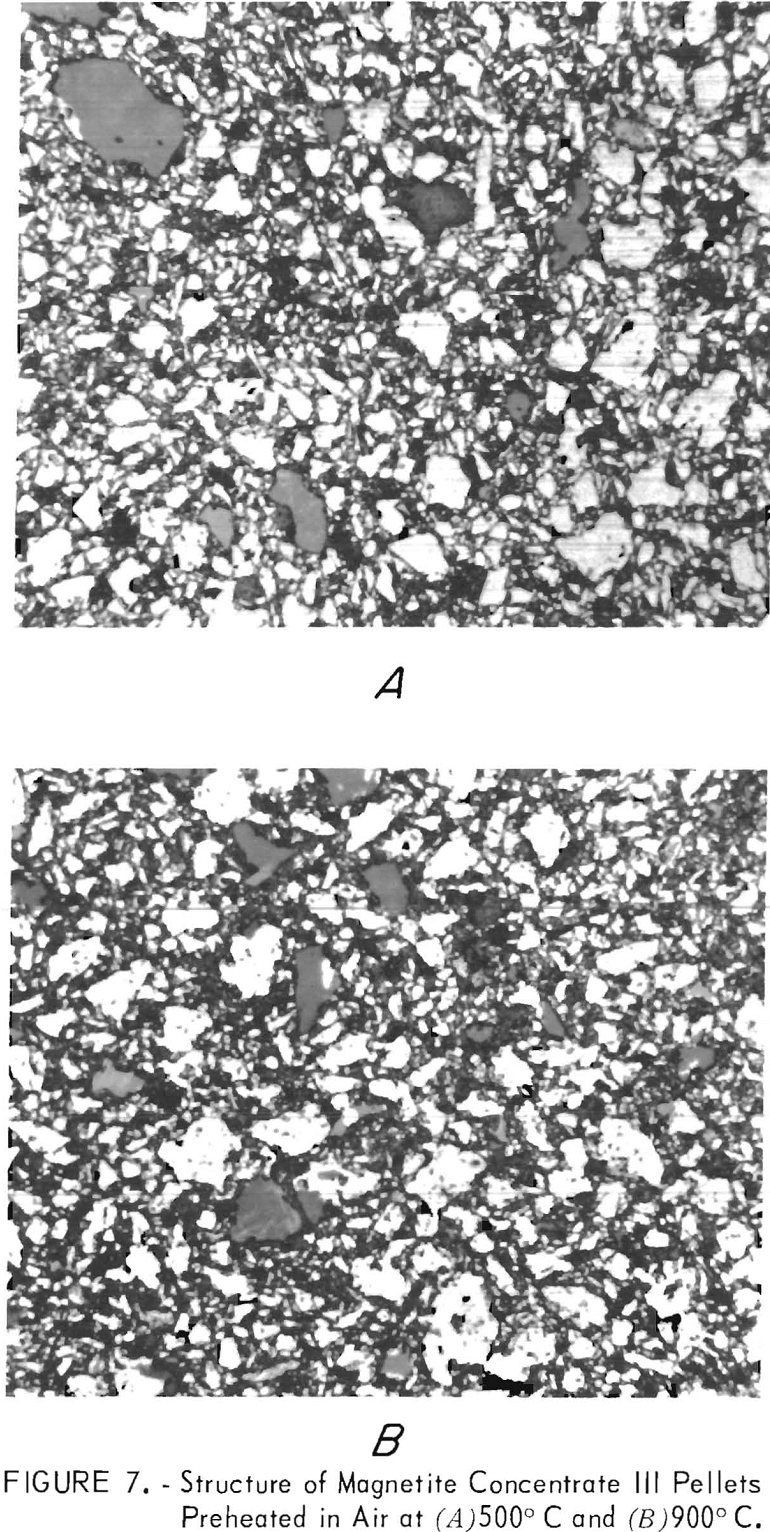
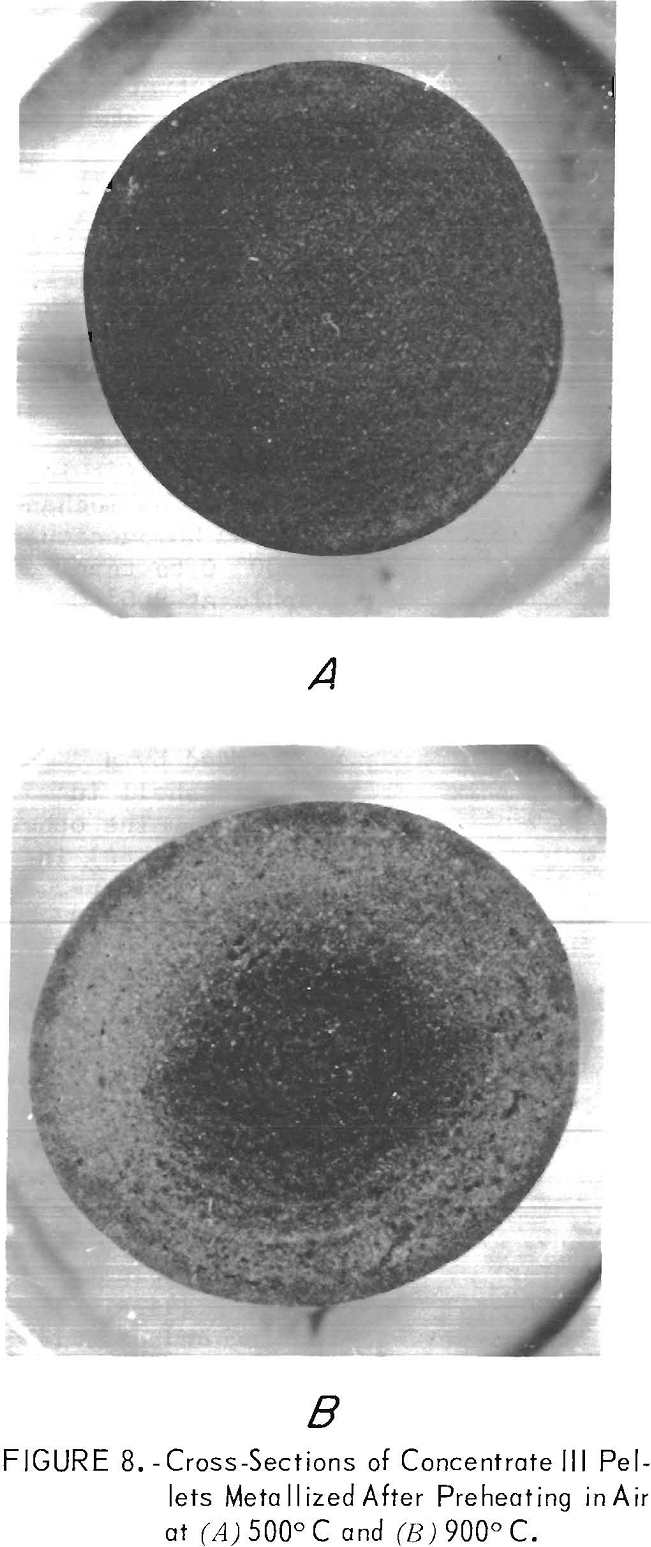
Petrographic inspection of magnetite concentrate III pellets preheated at 500° and 900° C (figs. 7A and 7B) failed to show any large changes in the physical structures, other than oxidation to hematite. However, inspection of the metallized version of the same pellets revealed a shift in the reduction mechanism from homogeneous at 500° C to topographic at 900° C (figs. 8A and 8B). This is evidenced by appearance of the dark wustite core surrounded by a metallic shell in figure 8B. The other pellet (fig. 8A) is a mixture of partially metallized grains of iron and wustite, an indication metallization was fairly homogeneous throughout the pellet. This shift from homogeneous to topographic reduction is typical of a pellet that has become less permeable to reducing gases. The bonding and assimilation of the fine particles by the larger particles lowers the oxide surface area exposed to the reducing gases and apparently inhibits the diffusion of reduction gases and products in and out of the pellet.
Effect of Preheating Upon Pellet Properties
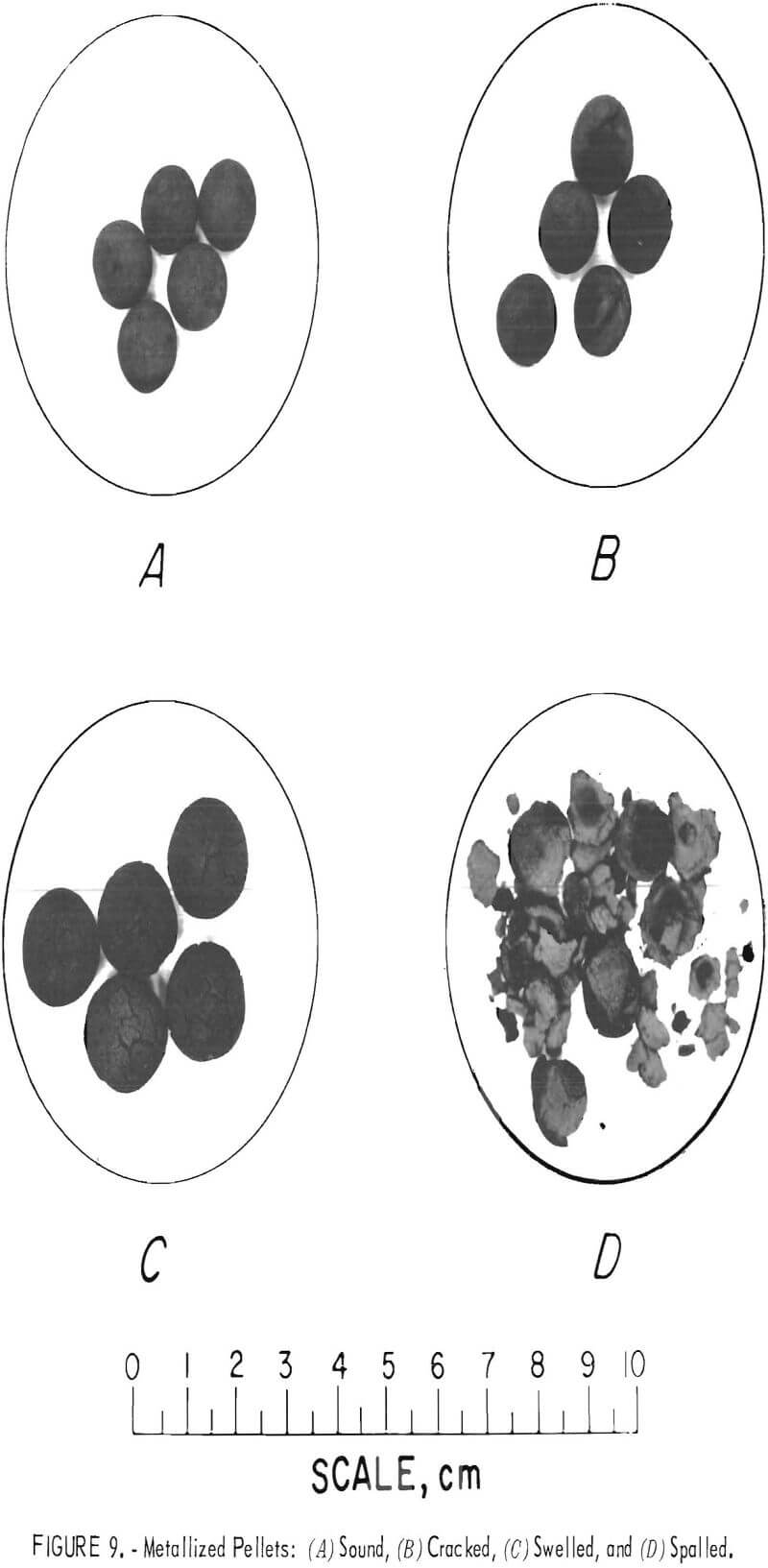
The prereduced pellet product must be capable of withstanding normal handling and transportation. Therefore, if preheating adversely affects the final physical properties of the pellets to the extent they are no longer satisfactory for iron-or steelmaking, any advantages gained have been effectively nullified. Cracking, swelling, and spalling are associated with reduction of indurated (fully or partially) pellets. Figure 9 shows a view of metallized pellets in various stages of disintegration.
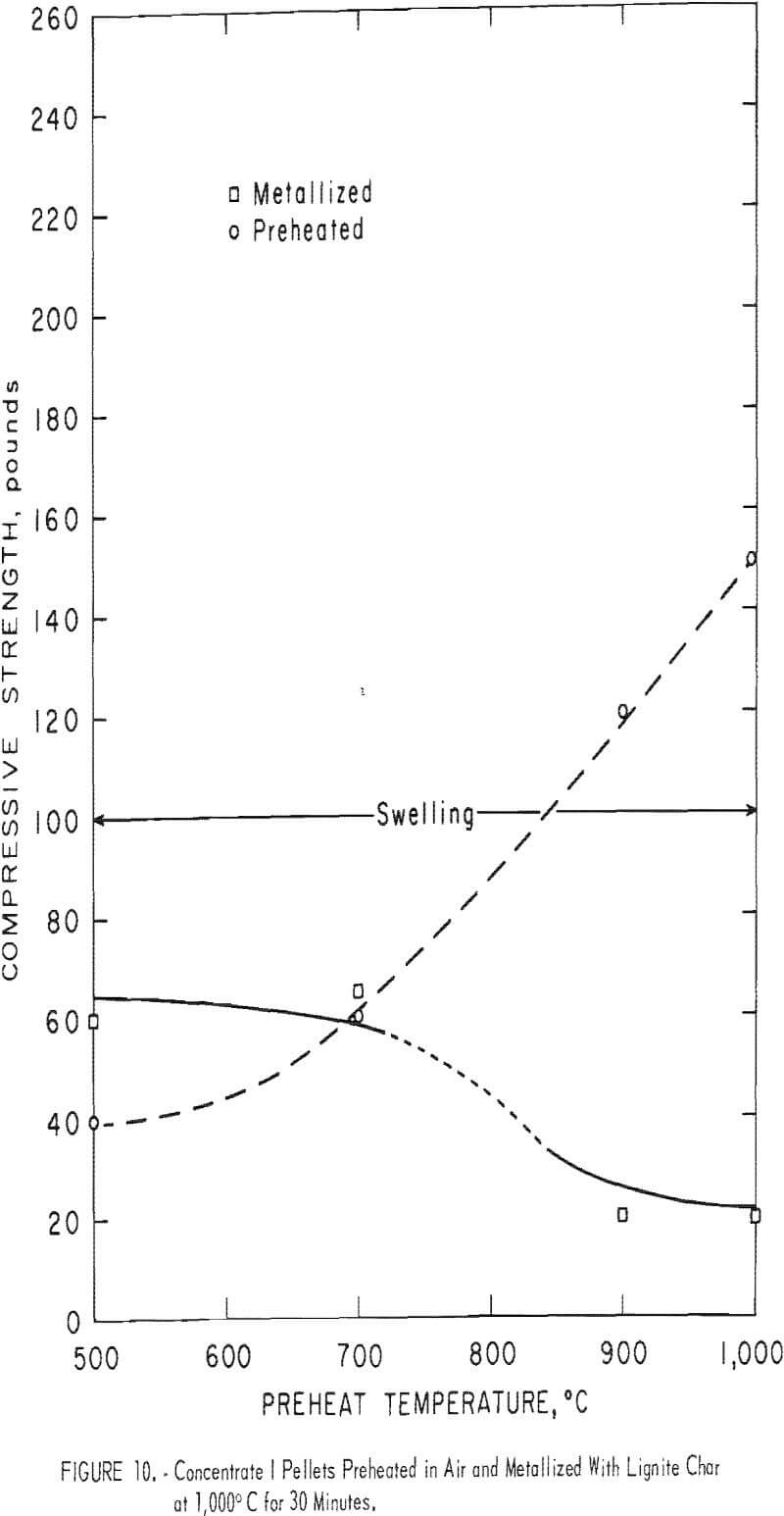
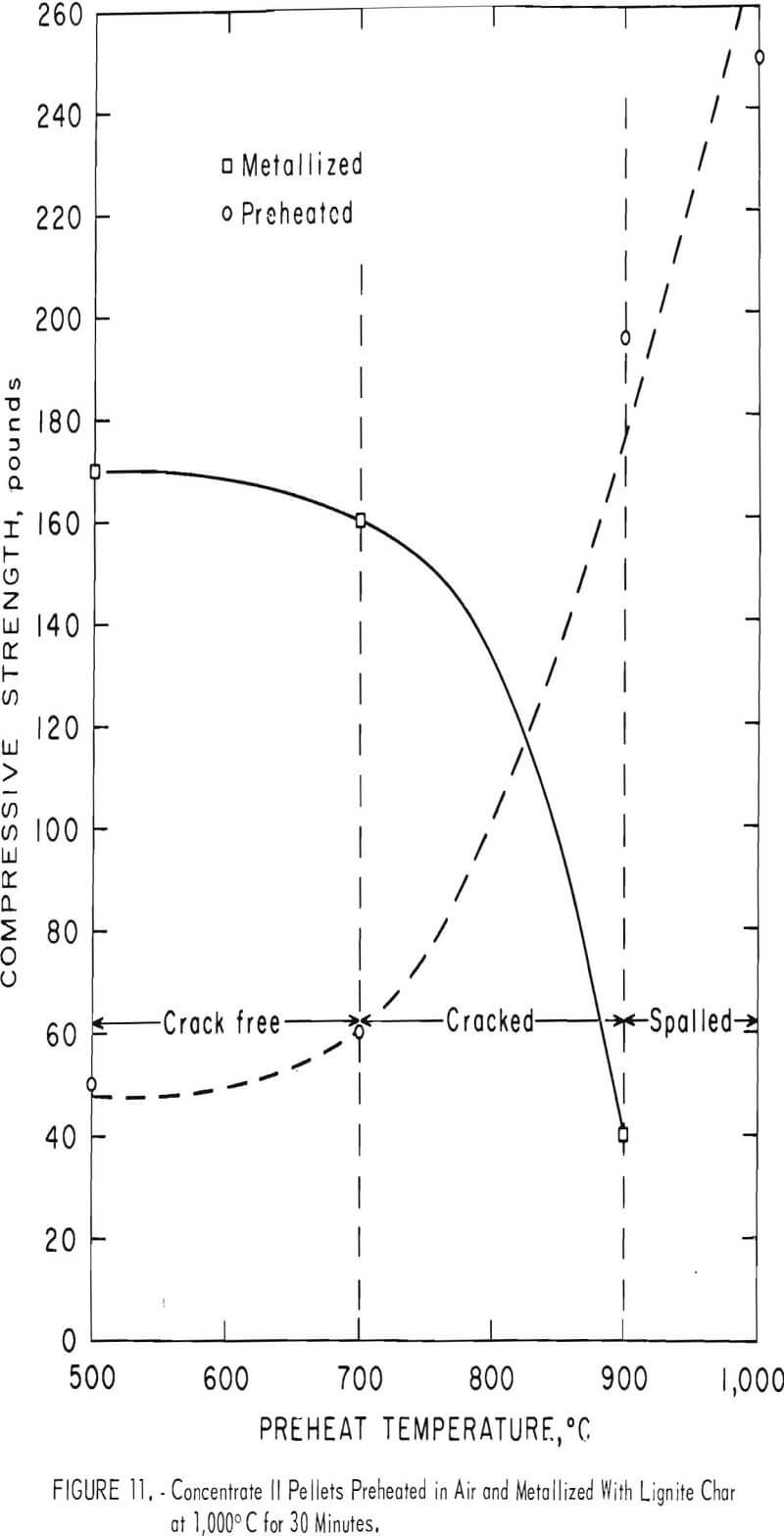
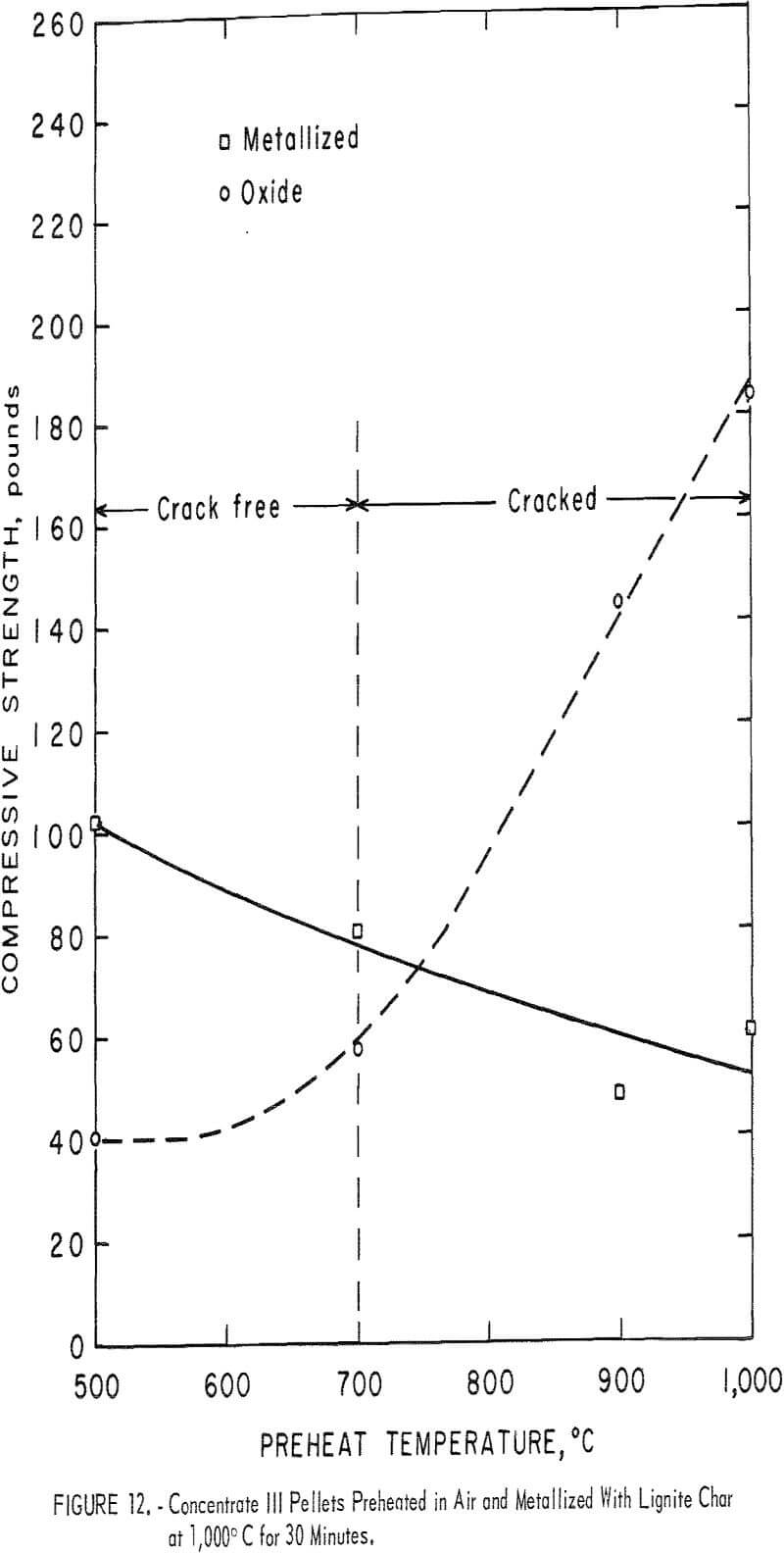
Figures 10, 11, and 12 illustrate the results obtained for the three magnetite concentrates (I-III). The room temperature compressive strength for both the preheated and the metallized pellets are plotted as a function of the preheat temperature. Also shown is a general indication of the condition of the metallized pellets, such as cracked or spalled.
Concentrates II and III (both Mesabi Range magnetites) behaved quite similarly during preheating (figs. 11 and 12). Both gained compressive strength rapidly in air above 700° C and the gain continued steadily up to 1,000° C, the maximum temperature used. Although no cracks were observed in any of the preheated balls, the figures show when preheat temperatures above 700° C were used, reduction cracks formed in the metallized pellets.
Concentrate III pellets, although cracked at preheat temperatures above 700° C, continued to remain intact up to 1,000° C pre-heat temperatures, whereas concentrate II pellets showed a distinct tendency to spall and disintegrate above 900° C preheat temperatures. In fact, the
destruction of the pellets was so complete that no metallized sample was available for a compression test at the 1,000° C preheat.
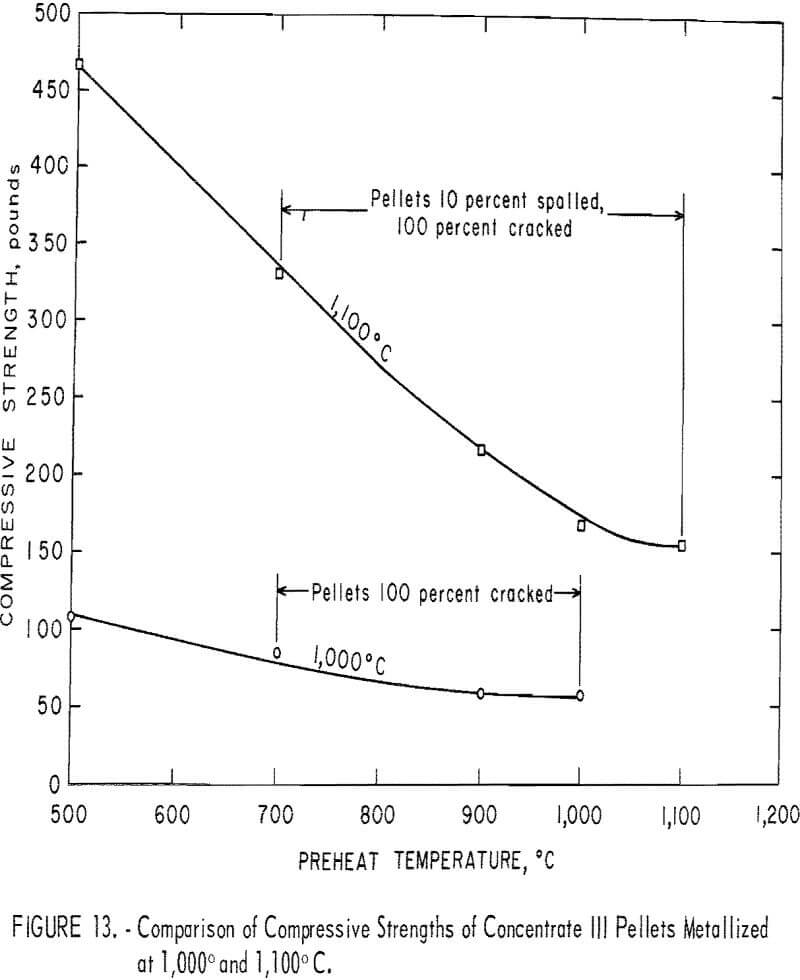
To determine the effects of a higher metallization temperature on the final compressive strength, concentrate III pellets were preheated in the usual manner, but were metallized at 1,100° C rather than at 1,000° C. The metallized compressive strength of these pellets along with the 1,000° C results are presented for comparison in figure 13. The large difference in compressive strength at the 500° C preheat temperature, 110 lb at 1,000° C and 460 lb at 1,100° C, steadily diminishes until at a 1,000° C preheat there is only a 100-lb separation between the curves. Cracking and spalling have essentially eliminated most of the gains from the higher metallization temperature.
Concentrate I (a Missouri magnetite) behaved quite similarly to concentrates II and III under preheating conditions; however, the pellets suffered severe ‘reduction swelling, resulting in soft, weak pellets. No satisfactory pellets were produced from any of the conditions used. However, reduction of concentrate I pellets previously fired at 1,300° C did result in strong adequate metallized pellets (360-lb compression strength).
Concentrate V (a hematite) also required complete induration at 1,300° C prior to metallization before resulting in an acceptable reduced product. Spalling was sufficiently severe at all preheat temperatures to prevent any physical evaluation of the metallized material. The pellets behaved normally during preheating and gave no indication of the subsequent physical deterioration under reducing conditions. The fully indurated (1,300° C) pellets were slightly cracked after 30 minutes metallization at 1,000° C, yielding a 93.7-percent-metallized product with a compression strength of 110 pounds.
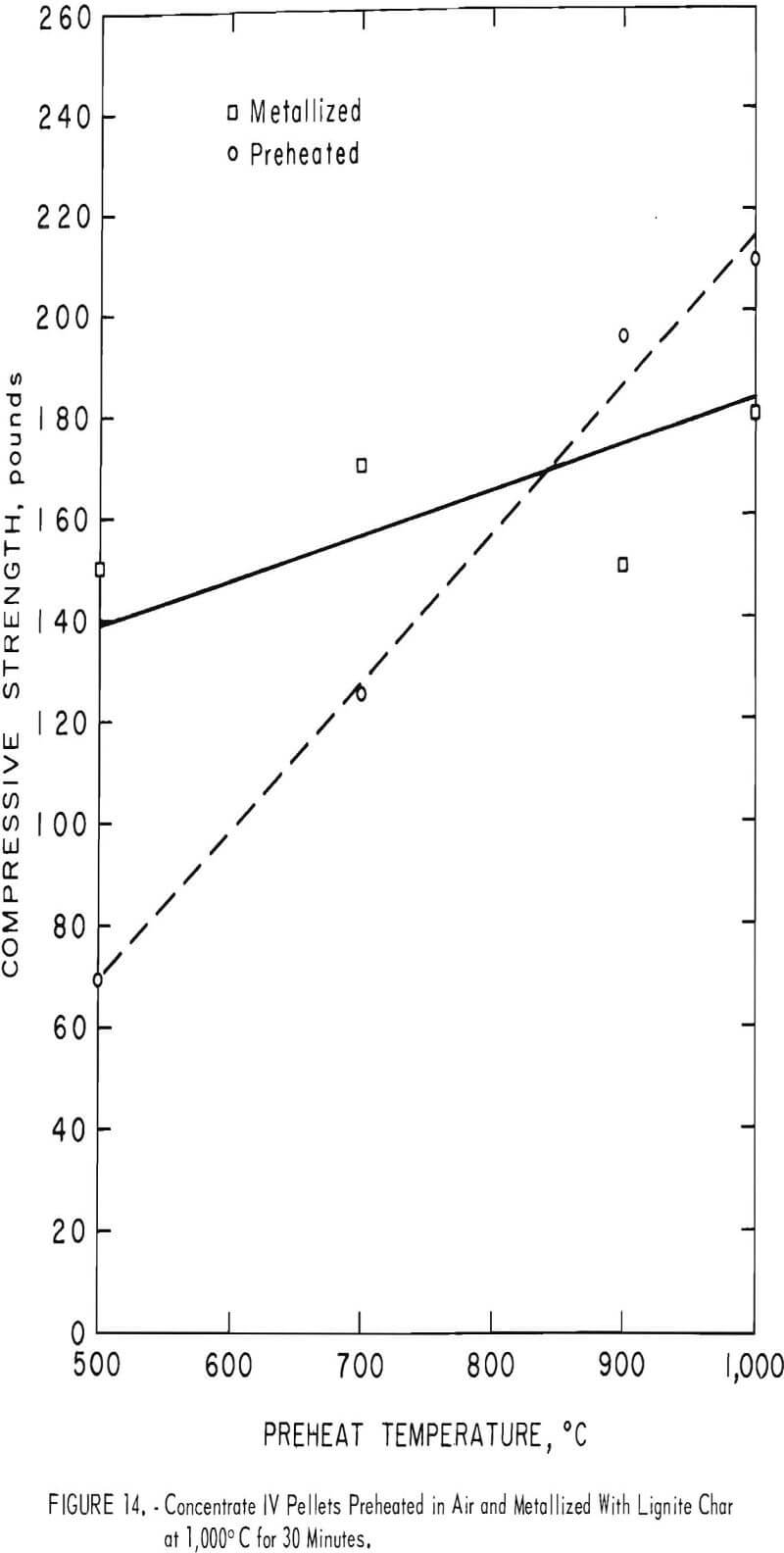
Concentrate IV, a Michigan hematite, produced exceptionally good metallized pellets at all preheat temperatures (fig. 14). It was the only concentrate to exhibit increasing metallized compressive strength with increasing preheat temperature. No cracking, spalling, or swelling occurred at any preheat temperature. The high silica content of this sample was unfortunate from a practical standpoint; it is, however, one of the better U.S. hematite concentrates marketed in the form of pellets. Of the four concentrates tested, concentrate IV produced the best pellets from compression strength and general physical appearance.
Conclusion
The results obtained from subjecting the five concentrates to a laboratory process involving the unit operation of balling, drying, preheating, and metallization indicated each concentrate had its own characteristics which could in part dictate limitations upon the process used to produce metallized pellets from it. Although concentrates II and III were both from the Mesabi Range, concentrate III pellets could withstand considerable preheating and still remain intact, while concentrate II pellets could not withstand preheating beyond 700° C and consistently produce acceptable pellets. It could be generalized that the best preheat conditions for these concentrates would involve a preheat to 700° C or less followed by metallization between 1,000° and 1,100° C.
On the other hand, concentrate I required complete induration at 1,200° to 1,300° C to prevent swelling during metallization. Under these limitations, a reactor capable of producing indurated oxide pellets would have to be used in conjunction with a metallizing reactor.
Concentrate IV, a high-grade earthy hematite screen concentrate, could be processed utilizing any preheat temperature up to and including the metallization temperature. However, there is no need to produce a fully indurated pellet prior to metallization.
Concentrate V also required a complete induration before metallization to yield an intact metallized product. At preheat temperatures of 1,000° C and below, the pellets completely disintegrated during reduction.
At least one general statement can be made about preheating of pellets. The specific necessity to test a given concentrate and adopt a process that will produce acceptable pellets is evident. Although considerable effort was made to detect any chemical or mineralogical difference between the magnetites, none could be found. At least at the present level of agglomeration metallurgy, the science of solid-solid reactions is such that the interaction of complex and impure systems cannot he predicted. Within the scope of this investigation, the tools and techniques were not at hand to evaluate the effects of impurities contained within the lattice of the matrix minerals, but the results do indicate that the effects of trace components can be highly significant and that further extensive investigations, employing sophisticated technology, will be required before the science of solid state reactions in impure systems will be fully understood.
