Table of Contents
In spite of the importance of the presence of pyrite in the flotation of sulphide ores, not many studies have been made from the electrochemical point of view. It is generally recognized that the oxidation of sulphide minerals is one of the most important factors among those which affect the floatability of sulphide minerals. The concept that the presence of oxygen is essential to the flotation of sulphide minerals is supported by numerous experimental results.
Electrochemistry of Pyrite
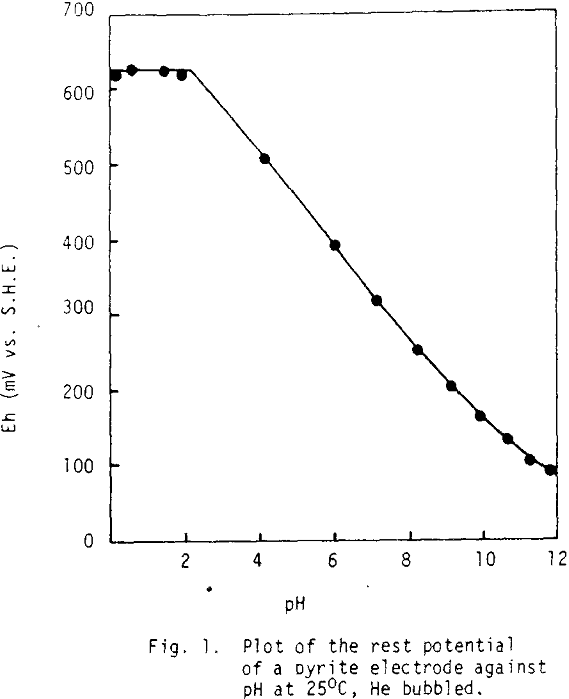
The rest potential of pyrite, corresponding to the open circuit (no net anodic or cathodic current) in 1NHClO4 was observed to be +0.62 volt vs. the standard hydrogen electrode (SHE). This value is consistent with those reported in literature’s, but is not expected, considering the equilibrium between pyrite and per chlorate solutions at pH=0. The equilibrium potential expected from the potential -pH diagram is at 0.35 volt at 1M Fe++ and would go to lower values in solutions more dilute in iron. The rest potential observed for pyrite is, in fact more noble than that observed for any other sulphide mineral or synthetically prepared sulphide matte.
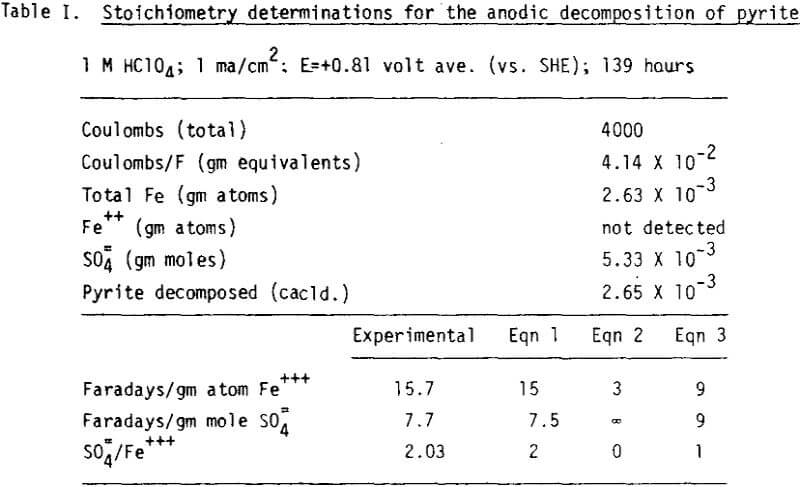
The stoichlometry of the anodic reaction is of interest at potentials below 1.0 volt, where oxygen discharge cannot occur.
FeS2 + 8H2O → Fe+++ + 2SO4= + 16H+ + 15e-
FeS2 → Fe+++ + 2S° + 3e-
FeS2 + 4H2O → Fe+++ + SO4= + S° + 8H+ + 9e-
The film formed on pyrite leads to the presence of a passive film in air and may possibly be adsorbed oxygen or an oxygen-sulphur compound. Separate experiments have shown that it is not rapidly reformed in the presence of ferric ions, and can be destroyed on anodized pyrite without loss of a visible ferric oxide tarnish
Oxidation of Xanthate with or without Pyrite
Oxidation experiments were conducted with a potassium ethyl xanthate solution under both oxygen bubbling and oxygen pressure atmospheres at room temperature. Experiments in which ultraviolet measurements, due to xanthate ions, are involved show that the oxidation of xanthate under these conditions is rather slow, and the formation of oxidation products cannot be detected in ordinary flotation time. However, when pyrite is aerated in a solution containing xanthate, the formation of an oily film is observed. With xanthate, the oily film formed on such a mineral is identified as dixanthogen by means of infrared spectroscopy.
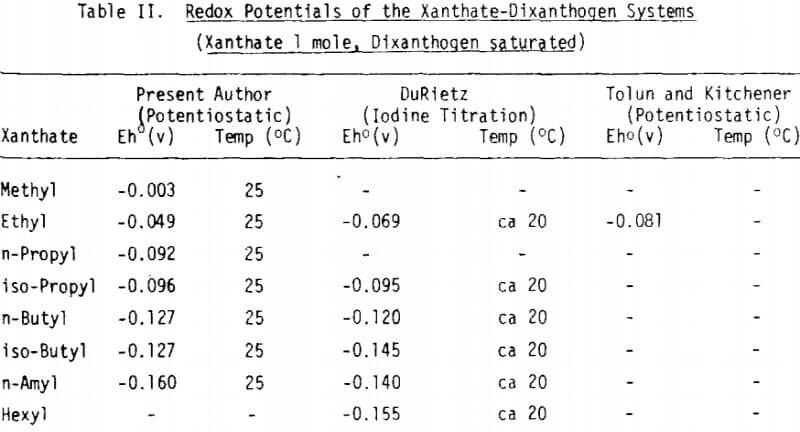
Measurements of the redox potential of xanthate-dixanthogen systems were attempted to get a fundamental background on xanthate oxidation. The redox potential for xanthate-dixanthogen systems increases linearly with increasing p[X-] (= -log\X-) value with a slope of about 60 mV/p[X-] in all cases. The redox potential of xanthate-dixanthogen becomes more negative with an increase in the carbon numbers of alkyl radicals.
Without xanthate, pyrite shows 0.17 V vs. S.H.E. of rest potential, but the value decreases with increasing xanthate concentration. The reducing behaviour of xanthate would lead to lower rest potentials in this case.
As mentioned before ordinary pyrite is in an ‘electrochemically passive’ state after exposure to air, and the film formed on pyrite that leads to passivity is present in air and may be adsorbed oxygen or an oxygen-sulphur compound.
The mechanism of dixanthogen adsorption on pyrite must satisfy all of these conditions. Assuming that adsorbed oxygen leads pyrite to the electrochemically passive state, the mechanism given by the following equation can be proposed,
½ O2 (ad) + 2X- + H2O ↔ X2 (ad) + 2OH-
Flotation of Complex Sulphide Ores Containing Pyrite
The selective flotation of pyritic complex sulphide ores is occasionally very difficult. The physical mixing of pyritic ore with other types of Cu-Pb-Zn-Fe complex sulphide ores sometimes results in a poor separation. The difficulties of selective flotation have been extensively examined as a function of activation of sulphide minerals with heavy metal salts formed by the oxidation of sulphide minerals. The oxidation of complex sulphide ore is found to be enhanced with the association of pyrite. This is observed in a daily operation as expected from fundamental research.
As mentioned before, pyrite has the highest rest potential among all the sulphide minerals. The condition of pyrite in its natural state may be regarded as electrochemically passive. The cathodic treatment can only generate a clean pyrite surface, and this has a thermodynamically predictable active potential. Since pyrite can exist in both active and passive forms, we may anticipate that when pyrite is in galvanic contact-with other sulphide minerals, the corrosion of the other sulphide minerals will be enhanced if the pyrite is in a passive condition, as in air.
The elemental sulphur formation reaction in an acidic solution may be favorable as expected from potential-pH diagrams for metal-sulphur-water systems. It can also be seen in those potential-pH diagrams that elemental sulphur is rather stable in an acidic or weakly acidic solution. Thus the presumption that elemental sulphur formed on sulphide minerals, which are in electric contact with pyrite in flotation pulp, plays an important part in the flotatlon of complex sulphide ore seems plausible.
In order to study the formation of elemental sulphur on sulphide minerals, four high-quality sulphide minerals; galena, sphalerite, chalcopyrite and pyrite, were subjected to oxidation experiments. An experiment with galena was performed with 20 g of mineral and 200 ml of solution adjusted pH at 2.0 with sulphuric acid by bubbling with compressed air. Minerals other than galena were added in quantities based on equal volumes of the solid sulphide phase.
The experimental results clearly show that the formation of elemental sulphur is accelerated in the presence of pyrite for all cases. The enhanced formation of elemental sulphur may be due to the galvanic action between pyrite and other sulphide minerals. As indicated by the previous study, elemental sulphur formed on sulphide minerals may cause difficulties in selective flotation of sulphide minerals.
The depression of pyrite with cyanide in the flotation system can be achieved in two ways. Cyanide is added to either freshly ground pyrite or xanthate-treated pyrite.
As retention time Increases, the concentration of cyanide ions decreases by the presence of pyrite. Using a cyanide ion electrode, it was found that the initial rate of cyanide consumption and the consumed amount of cyanide under
equilibrium condition are both proportional to the surface area of pyrite.
The rest potential shift of the pyrite electrode to the reducing side may be explained by either electrochemical activation of the pyrite surface due to the reduction of oxygen or adsorption of cyanogen or other cyanide derivatives on the pyrite surface. It has been shown that pyrite in an aqueous solution containing oxygen is in a passive state. This suggests that the decrease in rest potential of a pyrite electrode due to cyanide addition is predictable.
Studies of the depressive mechanism of pyrite with cyanide show that the removal of oxygen from the pyrite surface by cyanide may play the most important role of depression. Dixanthogen adsorbed on the pyrite surface is decomposed into mono-and di-thiocarbonates and thiocyanate by the addition of cyanide.