Table of Contents
Numerous low-grade gold-silver ore deposits throughout the Western United States have been developed in the past few years. Cyanide leaching and carbon adsorption are generally used to recover the gold. Precious metals and other adsorbed metals are eluted from the carbon with dilute caustic cyanide solutions. The elution process requires elevated temperatures and in some cases elevated pressures to increase elution rates. These elution procedures are not selective, and expensive high-temperature kilns are required to reactivate the carbon after several adsorption cycles.
Ion exchange is a well-developed technology and may have several advantages over activated carbon for recovering precious metals from cyanide solutions. Some of these advantages are (1) loading is faster, (2) gold-loading capacity is greater, (3) energy consumption in elution and regeneration is lower, (4) other valuable metals dissolved in the cyanide solution may be recovered when economics warrant, and (5) calcium and organics do not appear to poison resins to the same extent they do carbon. The first facility to use resin-in-pulp (RIP) ion-exchange technology commercially to recover gold from cyanide pulp was at the world’s largest gold mine, in Muruntau, U.S.S.R. Resin-in-pulp is now used widely for gold extraction in the U.S.S.R., and RIP processes offer definite economic advantages over carbon-in-pulp processes.
Both weak- and strong-base resins have been used to adsorb gold and other metals from cyanide solutions. Weak-base resins are generally more selective for precious metals but have lower loading capacities. Because protonation of the weak-base resin is required to extract anions, a pH of less than 10 is usually required. Currently there are no commercial weak-base resins that load well at pH values greater than 10; however, some experimental resins have shown good adsorption at pH values greater than 10.
A general mechanism for loading a weak-base resin follows:
Protonation:
RZNR2 + H+ X- ↔ RzNR2H+X-…………………………………………………………..(A)
Loading:
RzNR2H+X- + M(CN)2- ↔ RZNR2H+M(CN)2- + X-………………………………………..(B)
Rz, NR2, H+X-, and M(CN)2- represent the polymetric resin matrix, functional group, acid, and metal cyanide complex, respectively. Strong-base resins generally have higher loadings but are less selective for precious metals; however, they are much less affected by pH than weak-base resins. A general mechanism for loading a strong-base resin is
RzNR3+X- + M(CN)2- ↔ RZNR3+M(CN)2- + X-……………………………………………..(C)
Both resin types can be eluted at ambient temperatures and pressures. The weak-base resins may be eluted with dilute caustic solutions. The strong-base resins have been eluted with thiosulfate, acetone plus hydrochloric acid, ethylacetate plus nitric acid diluted with water, zinc cyanide, acid thiourea, and dimethyl formamide. All, except zinc cyanide have some disadvantages—usually fire hazard or noxious gas formation—or are uneconomical. General mechanisms for eluting weak- and strong-base resins follow:
Strong base:
RzNR3+M(CN)2- + X- ↔ RzNR3+X- + M(CN)2-…………………………………………….(D)
Weak base:
RzNR2H + M(CN)2- + X- ↔ RzNR2H+X- + M(CN)2-……………………………………(E)
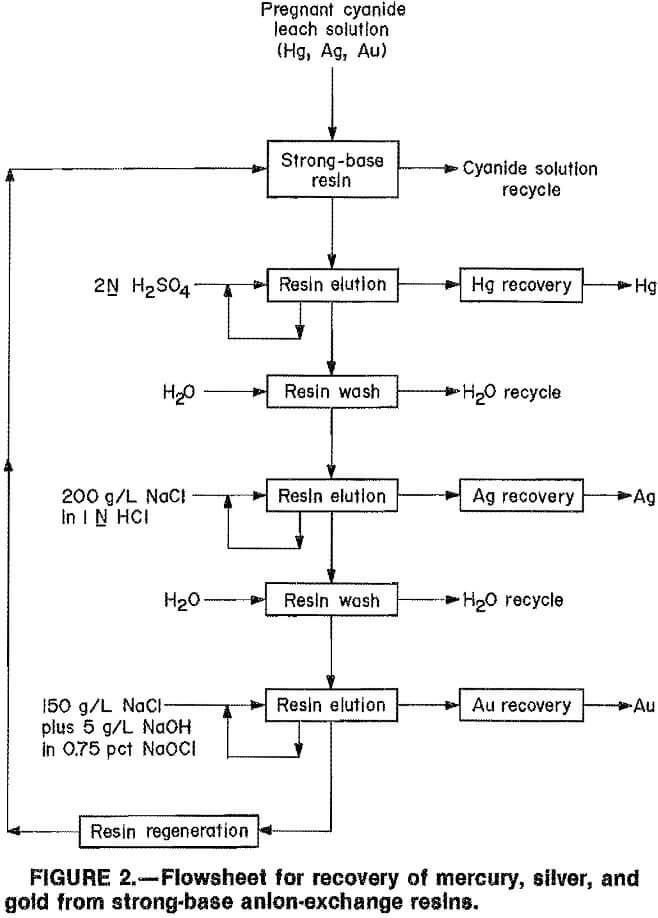
The flowsheet for resin-in-column is similar to that for carbon-in-column. The loading section is nearly identical, but the elution section does not require elevated temperatures, pressures, or expensive reactivation kilns.
The objectives of this Bureau of Mines investigation were to (1) investigate adsorption of precious metals from cyanide solutions with strong-base anion-exchange resins and (2) selectively elute mercury, silver, and gold from the resins, using inorganic aqueous eluants at ambient conditions.
Materials Equipment and Operating Procedure
Pregnant synthetic solutions prepared from reagent-grade mercury, silver, and gold chemicals were used to load resins. Feed solutions contained 5 ppm each of mercury, silver, and gold and about 0.5 g/L free cyanide; pH values were 10.5 to 11.4. Rohm and Haas IRA-430 and IRA-900 and Dow Chemical 21K, SMA-1, and SBR strong-base anion-exchange resins were used in this investigation. The IRA-430 and Dowex 21K resins have been used for many years for RIP in the uranium industry. The other resins tested have similar characteristics, and some are reported to be very resistant to physical breakdown and organic fouling. The resins consist of divinyl-benzene cross-linked with styrene and a quaternary ammonium functional group. Resin particle sizes were plus 20 mesh.
Resin loading was conducted by adding 2.5 mh of resin to 1 L of solution containing 5 mg each of mercury, silver, and gold and their radiosotopes to a plastic bottle. The bottle and contents were rotated or rolled on a set of horizontal rolls. After loading, the solution was removed from the resin by aspiration through a glass frit. The resin was washed with water, and the radioactivity was counted. Final resin loading was 145 oz/st dry weight (56.6 g/ft³ wet volume) of each metal.
Resins were eluted by rolling with 10 to 100 mL of prepared eluting solution for a specified time, aspirating the solution, washing with water, and counting the radioisotopes left on the resin. Elution and counting were repeated as needed. Solution analyses were accomplished by atomic adsorption or by counting radioisotopes in the solutions on a Tracor-Northern TN-1710 gamma counter. Radioisotopes analyses compared well with atomic adsorption analyses. Therefore, self-determination correction factors for different sample compositions and gamma-ray energies were not necessary.
Results and Discussion
Elution of Mercury and Precious Metals from Resins
The solubility of mercury cyanide in acids and of silver compounds in strong chlorides suggested that mercury and silver could be eluted from strong-base resins with dilute acids and strong chloride solutions, respectively. Gold be more strongly adsorbed on the resin than gold cyanide. Preliminary tests were conducted to elute (1) mercury with dilute H2SO4, HNO3, and HCl solutions, (2) silver with NaCl-HCl solutions, and (3) gold with NaOCl and NaClO4 solutions. The preliminary tests showed dilute H2SO4 elute mercury only, HNO3 eluted considerable silver with the mercury, and NaCl-HCl eluted silver and suppressed mercury elution. Therefore, additional testing was conducted with dilute H2SO4 for mercury recovery. Preliminary tests also showed good elution of silver with strong acid chlorides, and good gold elution with either NaOCl or NaClO4. Additional testing was conducted to determine the conditions for selective mercury, silver, and gold elution from five strong-base anion-exchange resins.
Mercury Elution
Mercury-, silver-, and gold-loaded IRA- 430 resin was eluted with increasing concentration of dilute H2SO4. Loaded resin was rolled 5 h with 100 mL of acid, the acid was removed, and elution was determined by isotope counting. The test results listed in table 1 show 0.2N to 2N H2SO4 effectively recovered 95 to 98 pct of the mercury in 5 h, only 2 to 9 pct of the silver, and less than 2 pct of the gold.
Silver Elution
Strong acid chloride solutions rapidly desorb silver from strong-base resins. Therefore, tests were conducted using 1N HCl containing increasing concentrations of NaCl. Figure 1 shows
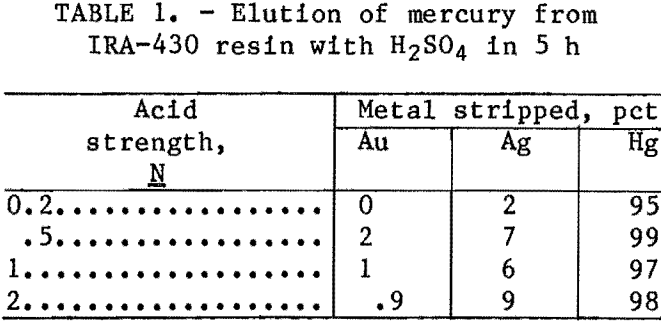
that silver elution increased from 30 to 98 pct in 6 h, as the concentration of chloride in the solution increased from 51 to 157 g/L (25 to 200 g/L NaCl).
Gold Elution
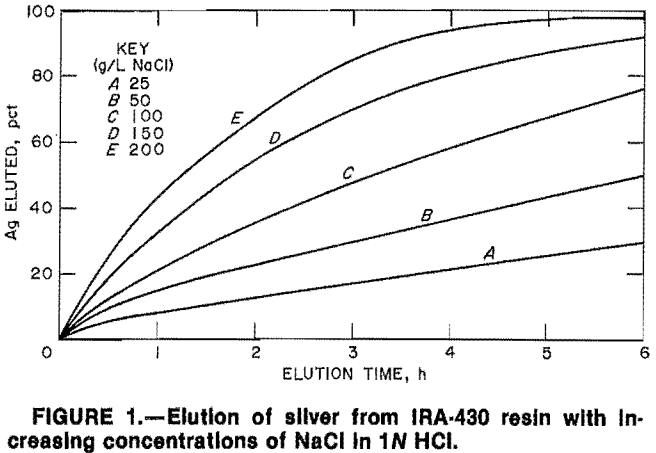
Preliminary testing indicated that alkaline hypochlorite and perchlorate elute mercury, silver, and gold from strong- base resins. Therefore, 1-h elution tests were conducted to assess the technique under more controlled conditions. Loaded IRA-900 was eluted for ten 1-h periods with a solution containing 150 g/L NaCl plus 5 g/L NaOH and 0.75 pct NaOCl. Recoveries were 87, 92, and 86 pct Hg, Ag, and Au, respectively. A mercury- and silver-free resin was similarly tested. Gold-loaded resin was contacted with solutions containing 0.25 and 0.75 pct NaOCl, 150 g/L NaCl, and 5 g/L NaOH. Gold elution was 78 pct in 2 h in the 0.75-pct solution and 89 pct in 16 h in the 0.25-pct solution. Gold-loaded Dowex SMA-1 and SBR resins were eluted with 0.5M NaClO4, and the IRA-900 resin was eluted with 0.5M, 1M, and 2M NaClO4. Results of the tests, given in table 2, show 85 to 93 pct of the gold was eluted from the resins in 10 h with 0.5M NaClO4. Using 2M NaClO4 94 pct was recovered in 5 h; 1M NaClO4 recovered 95 pct in 6 h. The tests show relatively good recovery of gold from strong-base resins in 5 to 10 h using either NaClO4 or NaOCl.
Sequential Elution of Mercury Silver and Gold
Elution procedures for mercury, silver, and gold discussed in previous sections suggest a possible flowsheet (fig. 2). To evaluate the elution scheme, loaded strong-base resins were contacted with 2N H2SO4 to elute mercury, with 200 g/L NaCl in 1N HCl to elute silver, and with 0.75-pct NaOCl In 150 g/L NaCl plus 5 g/L NaOH or 2M NaClO4 to elute gold. Two series of tests were conducted.
Results of the first test series using the batch elution method are given in table 3. These tests show 100 pct of the mercury was eluted from IRA-900, IRA-430,
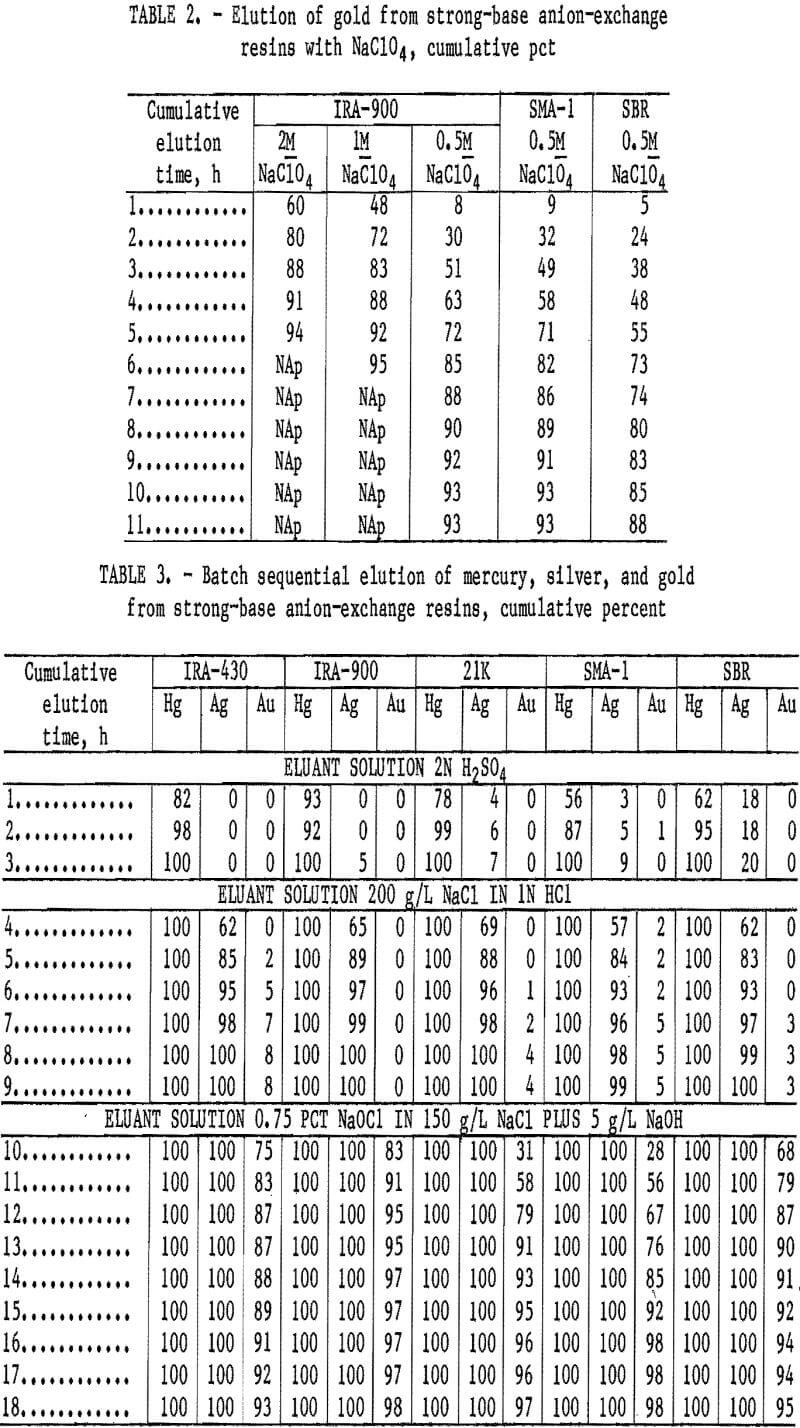
Dowex 21K, Dowex SMA-1, and Dowex SBR resins in 3 h. No gold was eluted from any of the resins with the mercury. No silver was eluted from the IRA-430 resin with the mercury, but 5 to 20 pct Ag was eluted from other resins in 2N H2SO4 solution. All of the remaining silver was eluted from all the resins with 200 g/L NaCl in 1N HCl. Gold elution ranged from 0 to 8 pct in the strong chloride solution and from 93 to 98 pct in 9 h in the 0.75-pct NaOCl solution. Silver and gold cyanides are not soluble in dilute acid solutions; therefore, any silver eluted with the mercury and gold eluted
with silver could be easily filtered from the solutions.
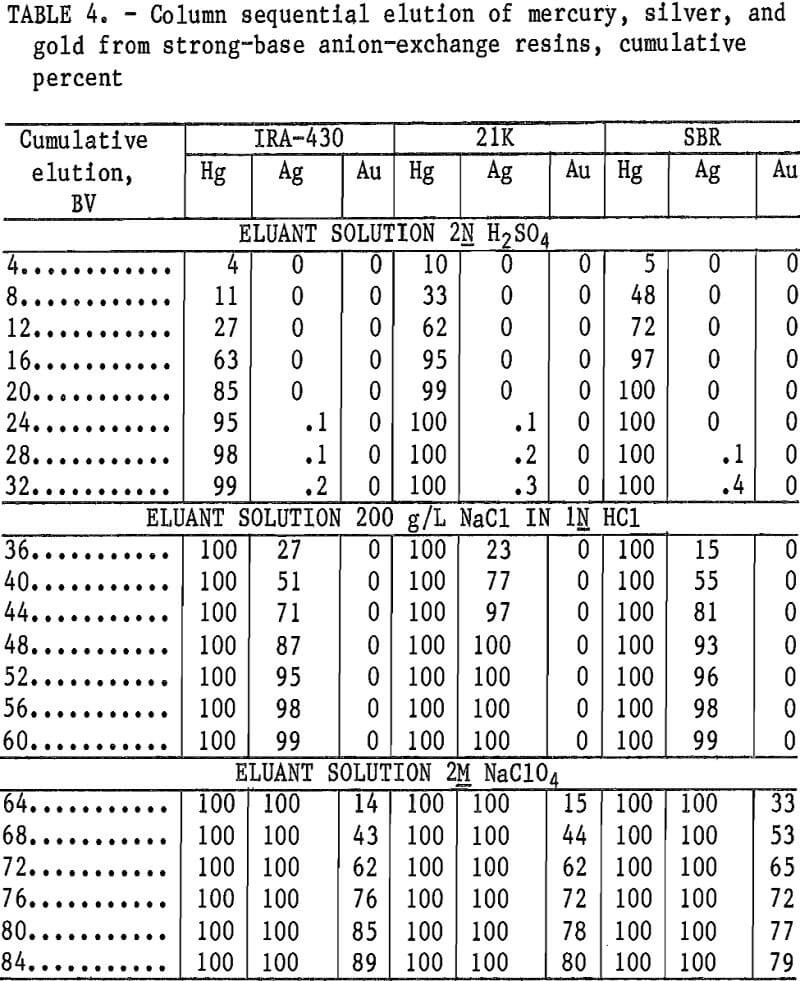
The second elution test series was conducted in columns using 25 mL of loaded resin. The IRA-430, Dowex 21K, and Dowex SBR resins were loaded with 145 oz/st of each metal, transferred to plastic tube columns, and eluted with solution downflow at 0.015 BV/min (0.375 mL/min). Cuts of 100 mL (4 BV) of the eluate were collected for assay. Test results given in table 4 show 99 to 100 pct of the mercury was eluted with 32 BV of 2N H2SO4, 99 to 100 pct of the silver was eluted with 28 BV of 200 g/L NaCl in 1N HCl, and 79 to 89 pct of the gold was eluted with 24 BV of 2M NaClO4. No silver or gold was eluted with the mercury, and no gold was eluted with the silver.
It was noted, however, that considerable AgCN precipitated in the columns after the H2SO4 elution, although none came through in the eluate. This indicates that an elution scheme to elute mercury with H2SO4 could not be performed in a fluidized-bed column unless a filter followed the mercury elution to remove the AgCN precipitate. It was also necessary to remove acid from the resin by washing or neutralizing prior to gold elution to prevent AuCN precipitation in the gold elute.
Metal Recovery
In addition to elution, research was conducted to recover precious metals from NaOCl and NaClO4 solutions. Both of these solutions elute mercury and silver with the gold if mercury and silver are not removed prior to gold removal from the resin. For those operations not containing mercury, the NaOCl or NaClO4 would be the preferred eluant. Therefore, tests were conducted at ambient temperature to recover the eluted metals by precipitation or electrowinning techniques.
Precipitation tests were conducted by adding slurried powdered zinc or aluminum to 1 L of eluate (150 g/L NaCI, 5 g/L NaOH, 0.8 pct NaOCl, or 2M NaClO4) containing 80 ppm Au, 12 ppm Ag, and 70 ppm Hg. Powdered zinc or aluminum, cleaned with NaOH, was added to the eluate and stirred. Samples of solution were taken every 10 min and analyzed. Over 90 pct of the mercury, silver, and gold were precipitated in 10 min from both the NaOCl and NaClO4 solutions with aluminum powder. Zinc powder precipitated 62 pct of the mercury, 64 pct of the silver, and 15 pct of the gold, in 30 min.
Electrolytic tests to recover the metal from the NaOCl and NaClO4 eluants were conducted in a compartmented flowthrough cell. The cell consisted of a Teflon filter cloth bag 1 in in diameter by 5 in long, filled with steel wool (cathode). The bag was placed in a 500-mL beaker containing a platinum screen anode. Solution, similar to that used in the precipitation tests, was pumped into the cell with gravity flow from the cell. Cell voltage was maintained at 3.5 to 4 V and ambient temperature. About 95 pct of the gold and silver were recovered
on the steel wood cathode from both the NaOCl and NaClO4 eluants. Mercury had previously been removed as described in earlier sections.
Conclusions
Five strong-base anion-exchange resins were tested for elution of mercury, silver, and gold with inorganic acids and salts. The resins tested included the Rohm and Haas resins IRA-430 and IRA-900, and the Dow Chemical resins 21K, SMA-1, and SBR. These resins were found to have similar elution characteristics with only minor differences. Resins loaded to 145 oz/st of each metal were eluted in batch and column tests.
Mercury elution of 99 to 100 pct was obtained in 3 h, with 2N H2SO4 in batch tests, and in 32 resin BV of acid in column tests. Silver elution of 99 to 100 pct was obtained in 6 h with 200 g/L NaCl in 1N HCl in batch tests, and in 28 resin BV of acid in column tests.
Gold elution of 93 to 98 pct was obtained in 9 h with 0.75 pct NaOCl plus 150 g NaCl and 5 g/L NaOH in batch tests. Gold elution of 94 to 95 pct was obtained in 6 h with 2M NaClO4 in batch tests, and 79- to 89-pct elution was obtained in 6 h in 24 resin BV in column tests.
In the batch elution tests, some silver was removed from the resins with H2SO4, and some gold was removed with strong chloride solutions; however, the silver and gold were not soluble in the dilute acids and could be easily filtered from the mercury solutions. In the column tests, no silver or gold was recovered with the mercury, and no gold was recovered with the silver.
Over 90 pct of the mercury, silver, and gold were recovered in 10 min from the NaOCl and NaClO4 eluants by precipitation with aluminum powder. Zinc powder was not as effective, with only 62 pct of the mercury, 64 pct of the silver, and 15 pct of the gold being precipitated in 30 min. In addition, 95 pct of the gold and silver were recovered from both the NaOCl and NaClO4 eluants by electrolysis using a Teflon-bagged cathode.
