In the froth flotation process, different types of surfactants are used to carry out specific and well defined functions. For example, an anionic surfactant such as xanthate is added as a collector in the flotation of sulphide minerals and adsorbs at the mineral/aqueous solution interface causing an hydrophobization effect. In addition, non-ionic surfactants are added and adsorb at the air/solution interface to control the frothing behaviour of the system.
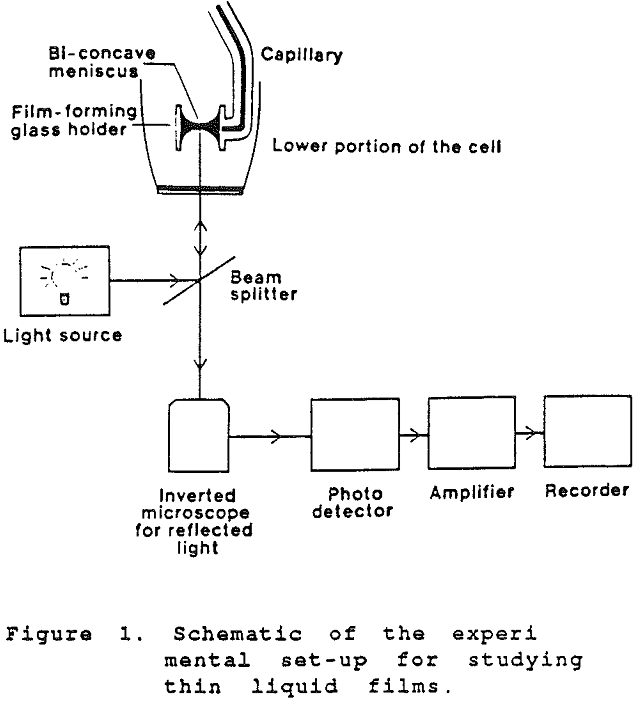
The film is formed between the tips of the menisci of a biconcave drop held in a cylindrical glass tube of 2.2 mm radius by sucking the liquid out of the drop. The amount of liquid in the biconcave drop is controlled by a teflon microsyringe, by which the radius of the film can be varied. The measuring cell is enclosed in a water jacket and maintained at a constant temperature (±0.1°C). The capillary pressure was determined by the method of capillary rise in a glass capillary.
The films form at an initial thickness that is much larger than that of the final state; thicknesses of up to 103 nm can be detected on the inter-ferograms . The thinning rate continuously slows down with time, i.e. decreasing thickness. After an “incubation” period during which the film exists as a thermodynamically unstable formation, kinetically stabilized by the Marangoni-Gibbs effect, an equilibrium state is gradually attained.
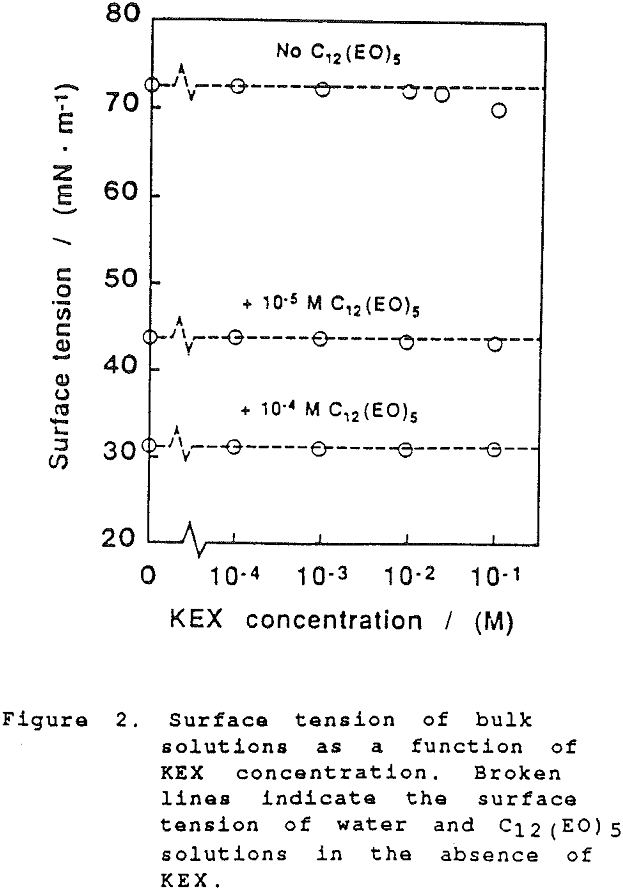
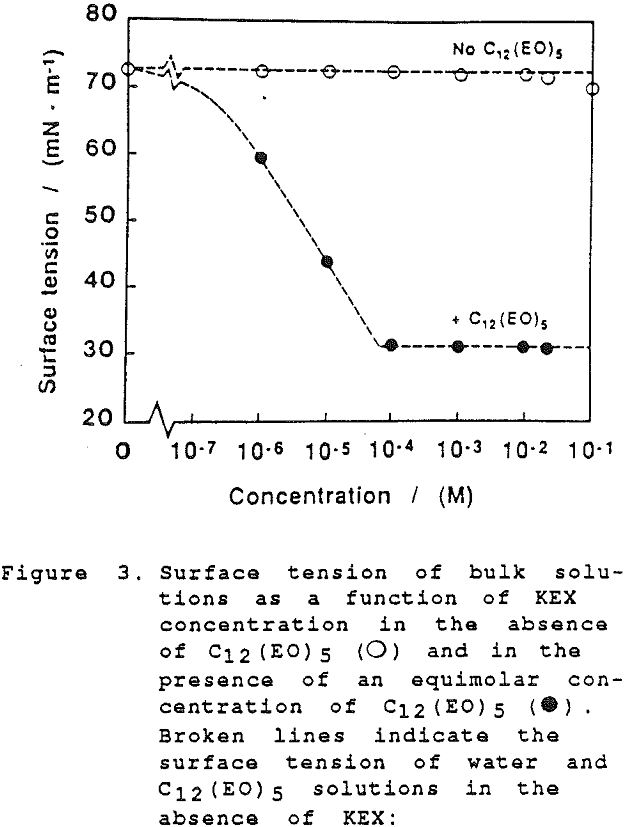
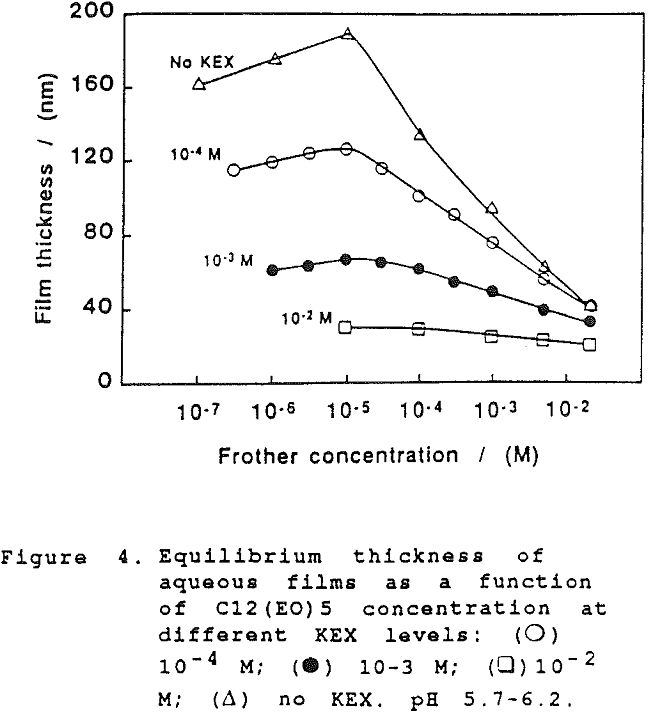
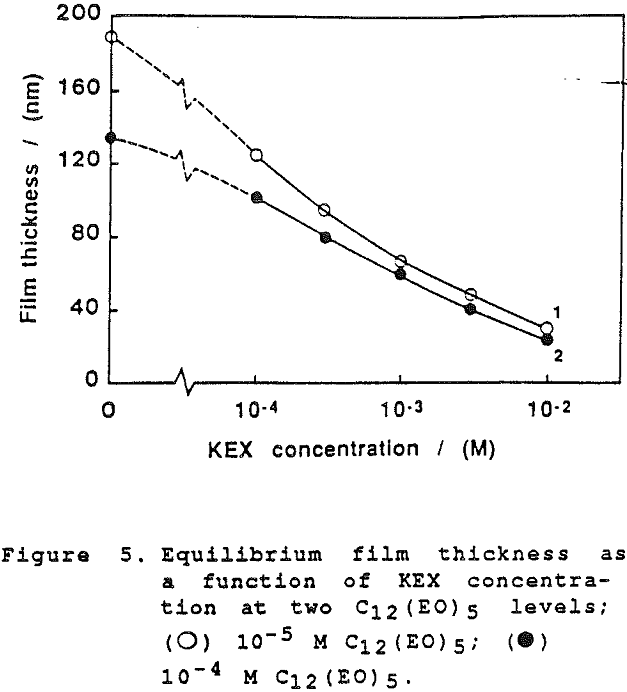
The thickness of equilibrium common thin films and black films from aqueous solutions of the non-ionic frother C12(Eo)5 were established over a wide range of frother concentrations (from 10 -7 to 2×10-² mol dm-³). The films were measured at the natural pH of the studied solutions, in the absence as well as in the presence of potassium ethylxanthate (KEX) of varying concentration (10 -4-10-² mol dm-³ KEX) .
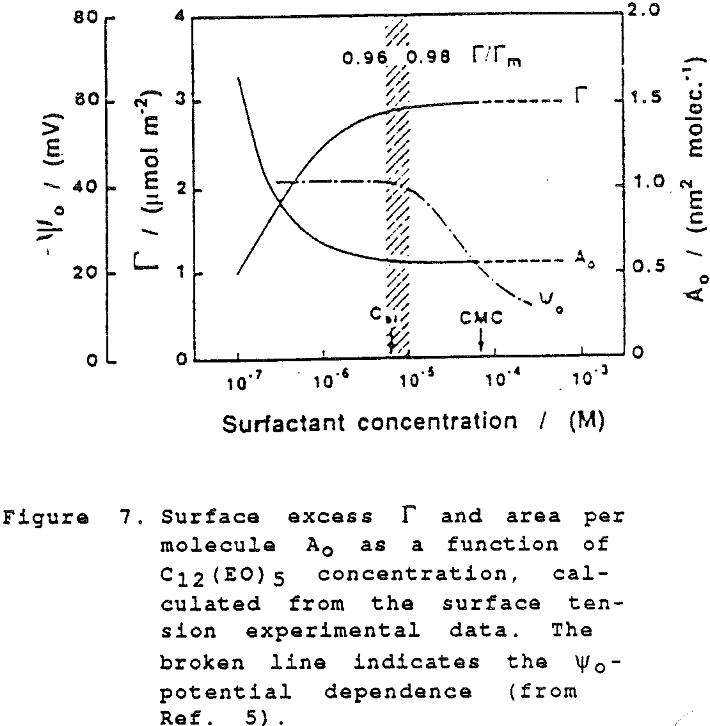
Although the surface is negatively charged, it is not at all necessary to assume that negative ions are preferentially expelled; simply, at these very high levels of adsorption there is not sufficient space at the interface for other species than the highly surface-active molecules of the non-ionic frother.
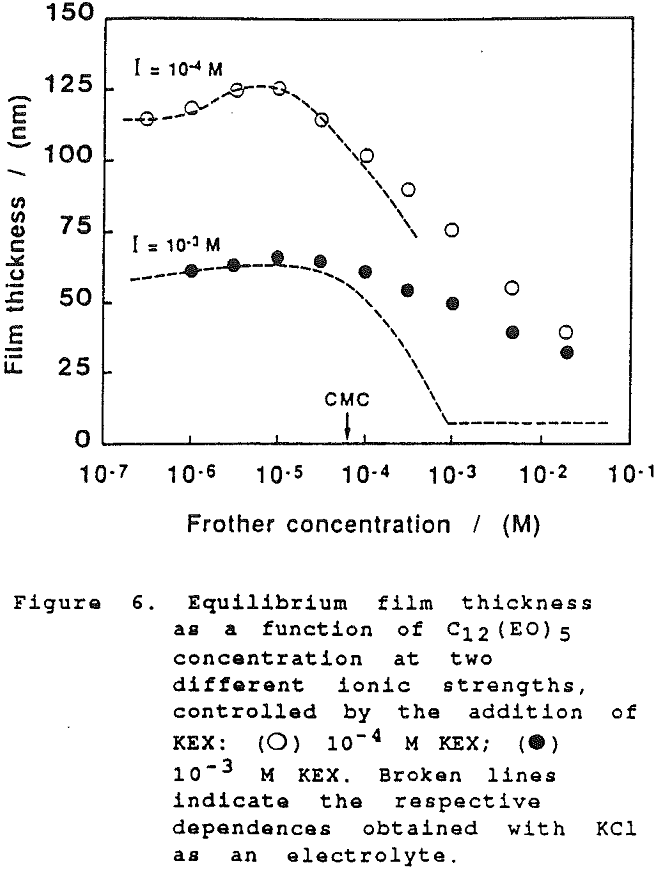
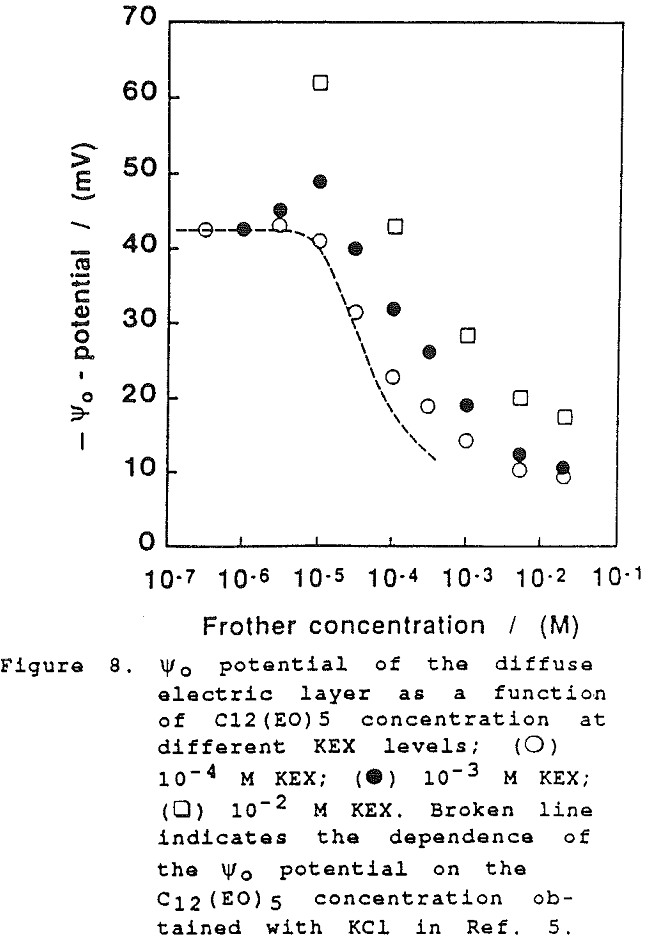
On further examination of our results in the present study, it would appear that the xanthate has a significant effect at high concentration on the film thickness. However, this can only be seen when comparing the dependence of the equilibrium thickness on the frother concentration at the same concentrations of xanthate and a common inorganic electrolyte, as shown in Fig. 6. (There again the results established with KCl are employed for comparison).