Flotation is a well known surface chemistry-based particulate materials separation process which utilizes differences in wettabilities of solids. The operating mechanism in the process is that finely divided particles are rendered hydrophobic by the adsorption of suitable flotation reagents, so that they are captured by the rising stream of bubbles which collide with them. Particles adhering to air bubbles are carried to the froth layer where they can be removed. The particles that are not sufficiently hydrophobic do not form a strong-enough bubble-particle bond and remain in the suspension, thus being rejected as tailings.
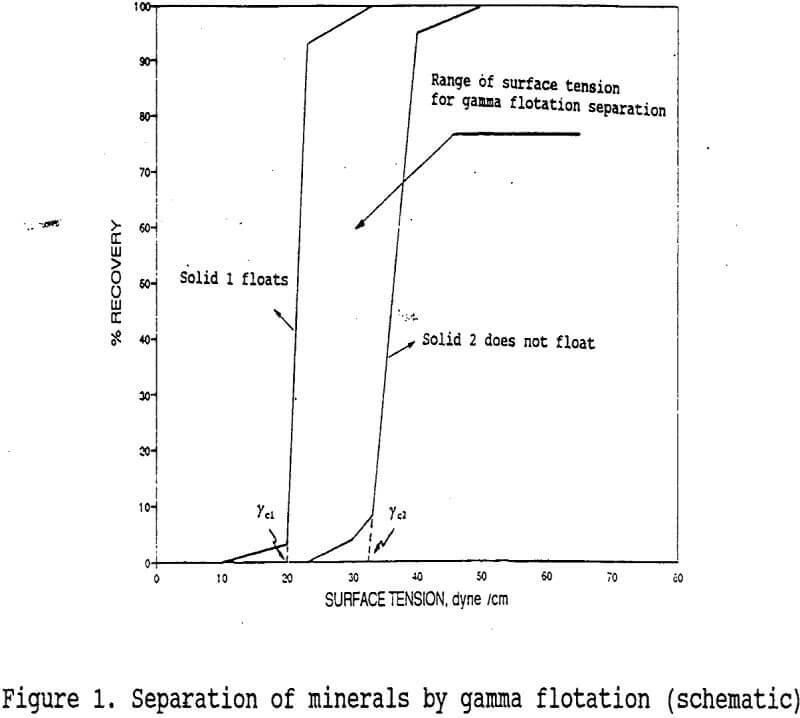
Wettability characteristics of mineral surfaces can be defined in terms of their critical surface tension of wetting values (γc) to achieve selectivity in flotation. The determination of the critical surface tension of wetting of a given mineral (in powder form) by flotation is obtained by plotting percent recovery versus surface tension, of solution (γLv) which gives a % R = 0 at γLV = γc. Here γLV is the surface tension of the solution forming the flotation pulp. It follows therefore, that a mineral is wetted by the solution if γLV < γc. Wettability control is achieved normally, by the use of conventional flotation reagents which act as collectors or depressants. Collectors reduce the γc of minerals to γc < γLV. Depressants on the other hand, induce the opposite effect i.e.: γc > γLv.
An alternative to the froth flotation process may be “ Gamma Flotation ” which has also been called “collectorless flotation”. It utilizes the γc differences of minerals as shown in figure 1. The process relies on controlling the surface tension of the flotation medium, where the γc of the minerals desired to float (solid 1) satisfies the condition γcl < γLV, while the minerals not desired to float (solid 2) satisfy the condition, γLV < γc. Therefore, the range of surface tension for separation schematically shown in figure 1 is used for separating solid 1 from solid 2 by the gamma flotation process.
In this paper we provide farther examples for the use of solution surface tension control for the separation of realgar, talc, stibnite, and sulfur from their mixtures without using conventional flotation collectors or frothers.
The choice of these solids (realgar, talc, sulfur, stibnite) is based on the fact that they represent low surface energy minerals whose γc values are less than 72 dyne/cm. They also report a wide range of wettability difference in terms of the γc values that make them separable by the gamma flotation process. Such low surface energy minerals that exhibit γc<γl=72 dyne/cm are conventionally known as “naturally hydrophobic”.
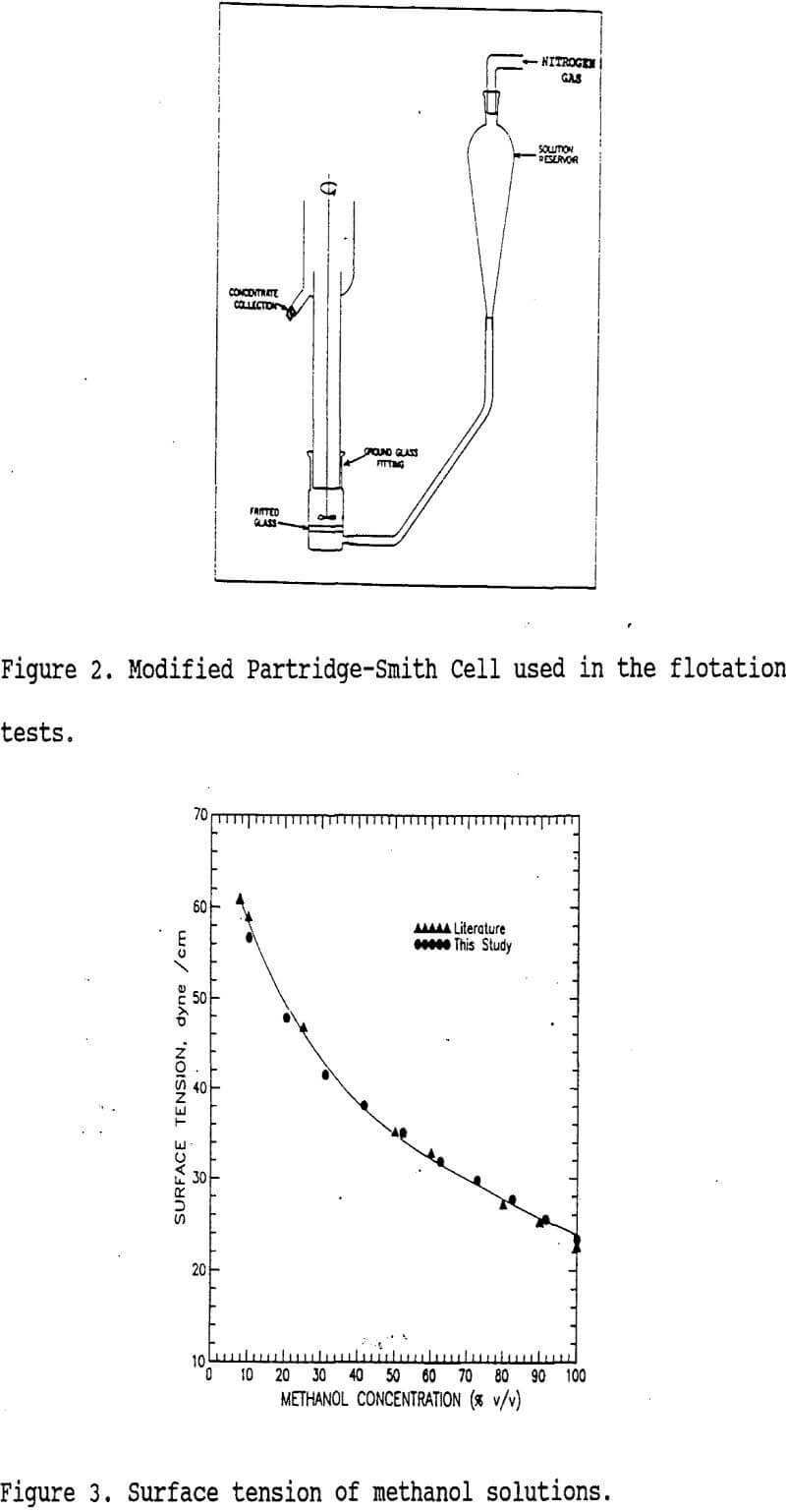
A modified Partridge-Smith cell (160 mL total volume) shown in figure 2 was used in the flotation experiments. Surface tensions of flotation media (γLV) were arranged by mixing methanol with water to obtain the range of 23.4 to 72.2 dyne/cm as shown in figure 3. The surface tensions of these solutions (γLV) were determined by the drop-weight method. The γc values of individual minerals were determined using the flotation technique described by Yarar and Kaoma. For separations, six grams of mineral mixtures (3g:3g) were conditioned in a solution of appropriate surface tension for one minute, and then three-minute flotations were performed under 30 cc/min nitrogen gas flow rate. The froth products were collected, filtered, dried and analyzed to obtain recoveries and grades of the minerals floated.