Table of Contents
As part of its effort to produce cell-grade alumina from clay, the Bureau of Mines investigated the hydrogen chloride gas-sparging crystallization of aluminum chloride hexahydrate (ACH) from aluminum chloride liquor, to provide information for optimizing the crystallization operation.
Four parameters important in controlling the crystallization of the aluminum chloride hexahydrate and the concentration of impurities, particularly phosphorus and magnesium, in ACH crystals are (1) aluminum chloride concentration in the feed solution to crystallization, (2) hydrogen chloride gas flow rate, (3) temperature, and (4) concentration of phosphorus and magnesium also in the feed solution. Parameters 1, 2, and 3 affect crystal formation. The combined effects are very important because the phosphorus and magnesium concentrate in the crystals during the early stages of crystal growth.
Aluminum chloride hexahydrate of cell-grade purity was crystallized from saturated aluminum chloride solutions containing less than 0.003 wt-pct P2O5 and 0.010 wt-pct MgO. Crystallization temperatures less than 60° C decreased crystal purity.
Bauxite, 93 pct of which is imported to the United States, is the only raw material used domestically for the production of alumina. As part of its effort to reduce U.S. dependence on foreign resources, the Bureau of Mines is investigating the use of domestic aluminous resources for producing feedstock to existing aluminum smelting capacity, particularly kaolinitic clay. Calcined kaolinitic clay contains 43 pct alumina, 52 pct silica, 3 pct titania, 1 pct iron oxide, and minor amounts (less than 0.1 pct) of magnesium, calcium, potassium, sodium, sulfate, phosphorus, and fluorine.
The Bureau of Mines is performing bench-scale and miniplant-scale research to improve processing methods and to obtain data considered essential during the preliminary design of a 25-ton-per-day alumina pilot plant for a hydrochloric acid leaching, gas-sparging crystallization technology. In the flowsheet developed for the proposed pilot plant, calcined kaolinitic clay is leached with a 5-pct stoichiometric excess of 26 wt-pct hydrochloric acid. Ninety-five pct of the alumina and varying amounts of associated elements are dissolved. The leach residue contains primarily silica, which is separated from the pregnant liquor. Iron in the liquor is removed by solvent extraction with tertiary amine organic extractant. Aluminum chloride hexahydrate is then precipitated from solution and purified by using two crystallization steps and hydrogen chloride gas for sparging. The washed ACH crystals are decomposed to reduction-grade alumina in a fluidized bed, and the HCl offgas is recycled.
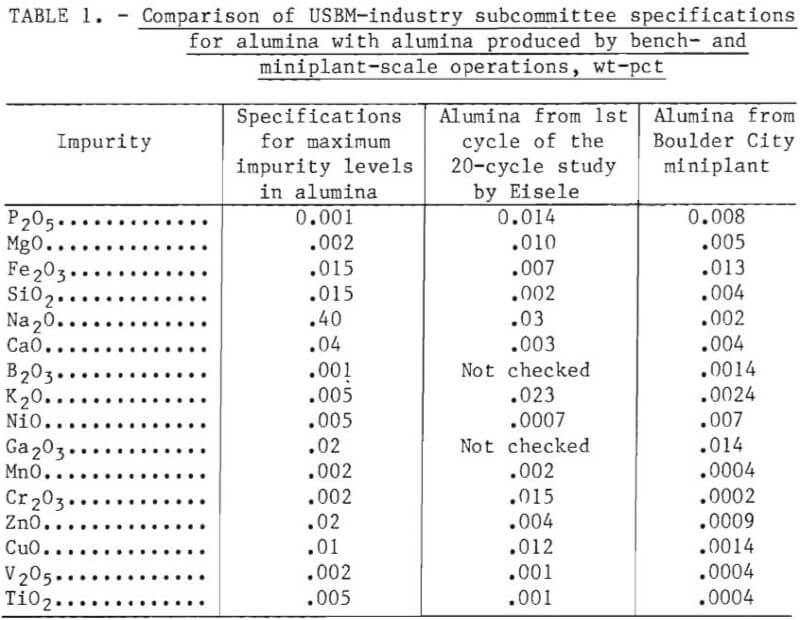
Table 1 shows a comparison of the alumina specifications set by a Bureau of Mines-industry subcommittee with the purity of alumina produced from the first cycle of leaching liquor of a bench-scale study and with alumina produced from first-cycle leaching liquor in a miniplant operation. The concentrations of most impurities in the alumina produced in the miniplant operation were less than specified maximums; phosphorus, magnesium, and nickel were the exceptions. The nickel content of the miniplant alumina slightly exceeded the specifications, probably because of corrosion of process equipment. The alumina from the bench-scale study contained high levels of phosphorus, magnesium, potassium, and chromium. Slowing the crystallization rate in subsequent experiments decreased the potassium and chromium content to acceptable levels. However, in both miniplant and bench-scale operation, phosphorus and magnesium always exceeded the specified maximum concentrations for alumina. For this reason a recrystallization step was included in the preliminary design of a 25-ton-per-day pilot plant by Kaiser Engineers, Inc.
If the separation of phosphorus and magnesium from ACH could be enhanced during the first crystallization step, the costly recrystallization step could be eliminated. A thorough study of the crystallization process was undertaken to investigate this possibility. Because the miniplant crystallizer operation was intended to generate engineering data, and because of the large scale of operation, detailed crystallization experiments could not be performed in the miniplant. Therefore, a detailed batch study of the crystallization process was carried out in bench-scale equipment.
Materials and Equipment
Top and side views of the batch, draft-tube crystallizer are illustrated in figure 1. The size was limited by the volume of the largest equipment easily handled in a laboratory fume hood. The crystallizer vessel was a 3-liter resin kettle with a four-port lid. Mixing was achieved with a polyethylene propeller rotating inside a glass draft tube. Three Teflon baffles centered the draft tube and prevented its rotation. Nitrogen and hydrogen chloride gases were metered into a packed column, where they were mixed and then injected into an aluminum chloride liquor one-half inch above the propeller blades. The crystallizer vessel was immersed in a water bath whose temperature was controlled within ±1° C by two immersion heaters. A glass-encased Chromel-Alurael thermocouple provided a signal for measuring the mother liquor temperature. The ACH crystals were separated from mother liquor by vacuum
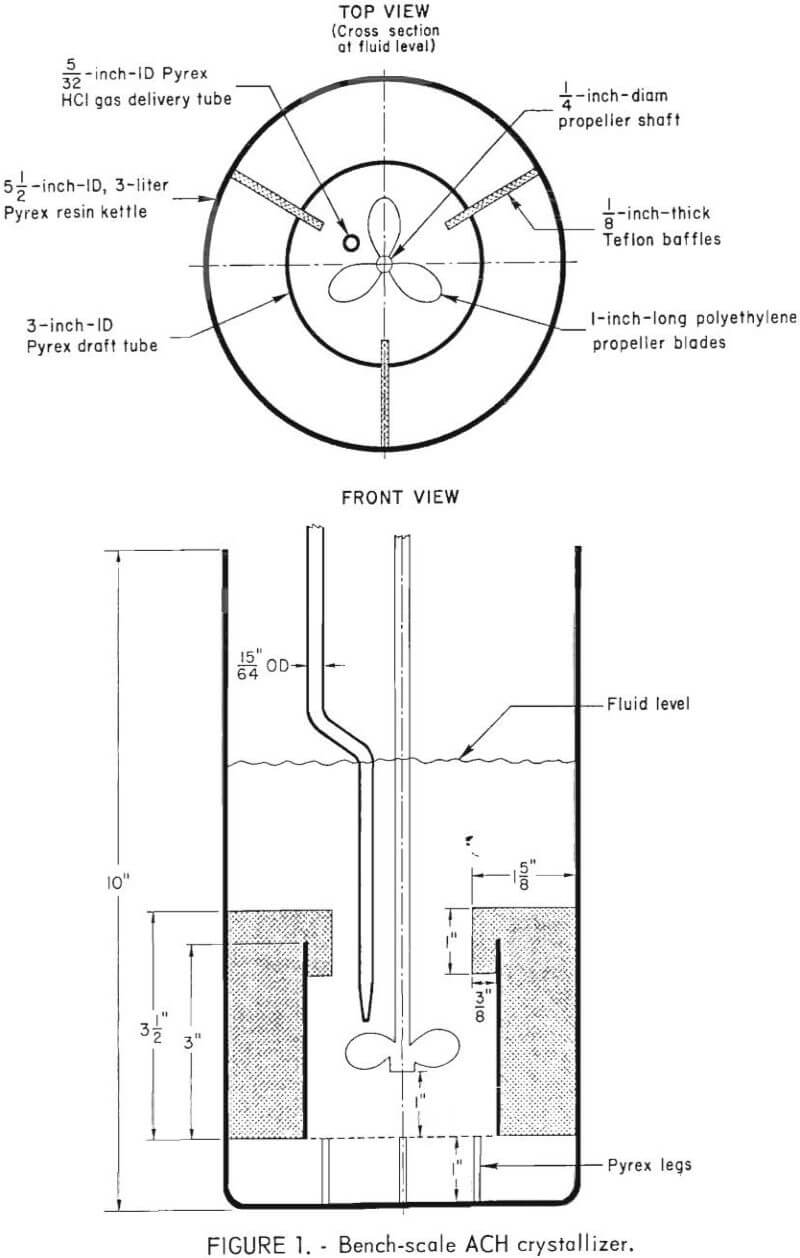
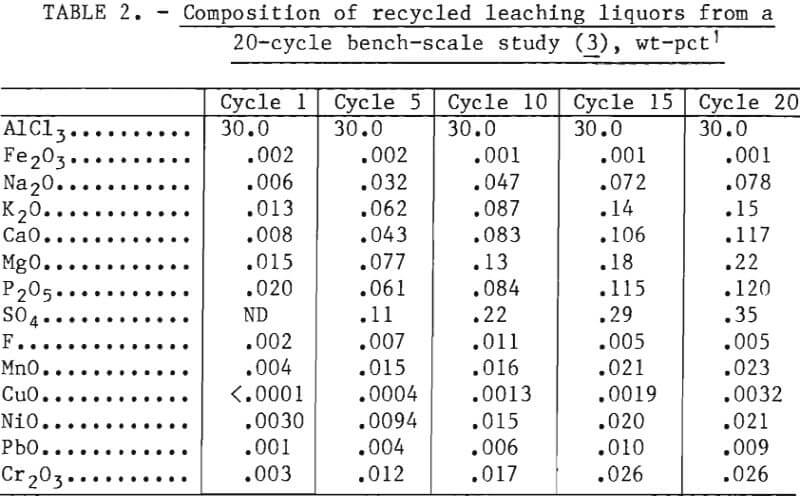
filtration through a coarse fritted glass disk in a Buchner funnel. After washing, the ACH crystals were dried with infrared heat lamps and constantly stirred during drying to prevent formation of crystal aggregates. The crystals were mechanically sieved through a set of U.S. Standard stainless steel screens to determine crystal size distribution. A sieve stack containing 28-, 35-, 48-, 65-, 100-, 200-, and 325-mesh screens was used. Crystallization tests were performed on aluminum chloride liquors of varying compositions. Synthetic liquors were prepared to match the compositions of selected cycles of the bench-scale recycle study and are listed in table 2. The use of actual leaching liquors was precluded because of the large number of different compositions required. Commercial ACH crystals were dissolved in distilled water, and selected compounds were added to bring the composition of impurities to that for the appropriate cycle. Most impurities were added as chlorides. Phosphorus, chromium, sulfate, and fluoride were supplied by phosphoric acid, potassium dichromate, sulfuric acid, and sodium fluoride, respectively.
Test Procedure
For each crystallization experiment, the crystallizer vessel was charged with 2.0 liters of aluminum chloride liquor. Aluminum chloride concentration in the liquor was 30 pct, and sparging with HCl was continued until the HCl concentration in the mother liquor was 26 pct, unless otherwise noted. The stirrer speed was set at 600 rpm, the water bath was heated to the desired temperature, and delivery of nitrogen carrier gas at 250 ml/min was started.
When the aluminum chloride liquor stabilized at the desired temperature, hydrogen chloride gas was added to the nitrogen gas at a flow rate of 1.3 l/min. Sparging continued until the hydrochloric acid concentration of the mother liquor was approximately 8 N (26-pct). The acid concentration was determined by decanting clear liquor from small samples of crystal slurry, adding potassium oxalate solution to an aliquot of the clear liquor to complex the aluminum, and titrating with 0.5 N sodium hydroxide. The ACH crystals were separated from the mother liquor by filtration. Entrained mother liquor was removed from the crystals by a displacement wash with 1.0 liter of 8 N HCl saturated with AlCl3 followed by a displacement wash, and two reslurry washes each with 1.0 liter of concentrated HCl. After drying the crystals, 100-gram splits for analysis were ground in a mortar and pestle to minus 100 mesh.
The aluminum chloride liquor, mother liquor, acid washes, and ACH crystals were analyzed for iron, sodium, potassium, calcium, magnesium, manganese, copper, nickel, lead, and chromium by atomic absorption spectroscopy, and for aluminum, phosphorus, sulfate, and fluorine by wet-chemical methods.
 Crystallizer Configuration and Gas Flow Rates
Crystallizer Configuration and Gas Flow Rates
The spacing and dimensions of the crystallizer draft tube and propeller were investigated and were found not to be major factors. The rotation of the propeller at 600 rpm insured vigorous vertical mixing by drawing the gas and liquor down through the center and up on the outside of the draft tube.
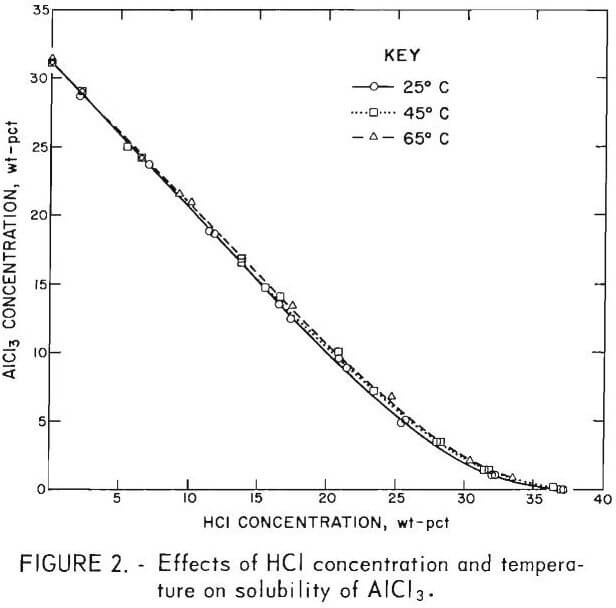
Hydrogen chloride sparging crystallization of ACH is based on the fact that AlCl3 solubility is low in concentrated HCl. Figure 2 summarizes results of a study that shows the effects of HCl concentration and temperature on the solubility of AlCl3. The solubility of AlCl3 at 65° C decreases from 31.2 wt-pct in water to 5.2 wt-pct in 26 wt-pct HCl.
Holding other parameters constant, gas flow rates were varied. The HCl flow rate was an important variable because doubling the HCl flow rate from 1.3 to 2.6 l/min doubled the phosphorus and magnesium concentration in the ACH crystals. Decreasing the flow rate below 1.3 l/min had no effect on crystal purity. It was important to prevent pockets of high HCl concentration from occurring, to minimize crystallization of ACH at the gas inlet. The most effective way was to mix the HCl with inert carrier gas and introduce the mixture just above the propeller blades.
Two sparger feed solutions generated at the Boulder City miniplant were crystallized in both the miniplant and the bench-scale crystallizer. The first sparger feed was a one-cycle leaching liquor generated by leaching calcined kaolinitic clay with 26 wt-pct HCl, removing iron by solvent extraction, and concentrating the AlCl3 to near saturation. The second sparger feed was made by dissolving ACH crystallized from the first sparger in deionized water. Both feed solutions were sparged at 60° C. The concentrations of impurities in ACH crystallized from both feeds are compared in table 3 and exhibit only minor differences. The results show that a batch, bench-scale crystallizer can be used to predict the formation and purification of crystals produced in the miniplant crystallizer.
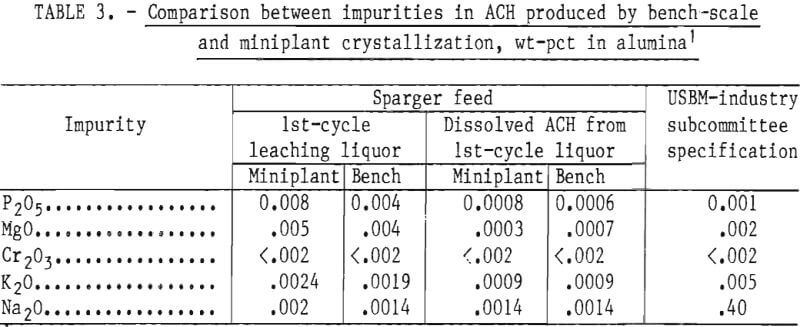
Aluminum Chloride Hexahydrate Yield as a Function of HCl Concentration
 The yield of ACH produced by sparging HCl into AlCl3 liquor depends on the AlCl3 concentration in the sparger feed and on the HCl concentration in the mother liquor. The equilibrium solubility data of Brown can be used to predict yields; this is shown as a dashed line in figure 3. The dots in figure 3 represent the ACH yields obtained from bench-scale crystallization experiments. There is close agreement between the experimental and predicted yields. An average of 80 pct of the AlCl3 was crystallized as ACH when 30 wt-pct AlCl3 liquor was sparged to 26 wt-pct HCl concentration.
The yield of ACH produced by sparging HCl into AlCl3 liquor depends on the AlCl3 concentration in the sparger feed and on the HCl concentration in the mother liquor. The equilibrium solubility data of Brown can be used to predict yields; this is shown as a dashed line in figure 3. The dots in figure 3 represent the ACH yields obtained from bench-scale crystallization experiments. There is close agreement between the experimental and predicted yields. An average of 80 pct of the AlCl3 was crystallized as ACH when 30 wt-pct AlCl3 liquor was sparged to 26 wt-pct HCl concentration.
Effects of Temperature on ACH Crystal Purity
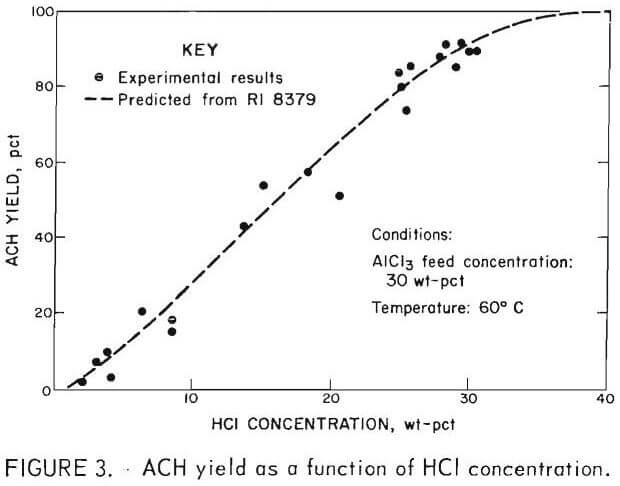
The effect of phosphorus and magnesium concentration in sparger feed on the concentration of phosphorus and magnesium in ACH crystals is plotted in figures 4 and 5 for temperatures of 20°, 40°, and 60° C. The advantage of operating at 60° C rather than 20° C or 40° C is emphasized. At 40° C and 60° C the rate of increase of phosphorus incorporated in the ACH diminishes at sparger feed concentrations greater than 0.12 pct. This fact has significance for a process utilizing a recrystallization step. The first crystallization can be made from an impure liquor (representing a small bleedstream) without significantly increasing the P2O5 content of the ACH recrystallized from the liquor. The diverging families of curves for phosphorus and magnesium show that ACH purity was increasingly dependent on temperature as the phosphorus and magnesium concentration in the sparger feed increased.
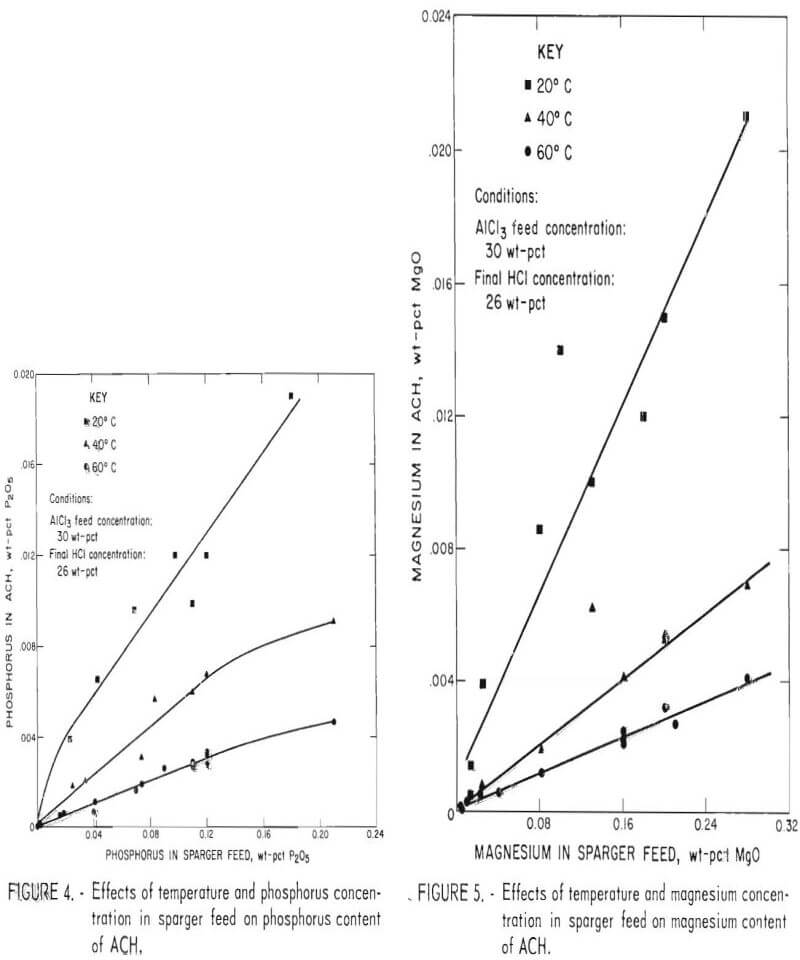
The effect of temperature on crystal size is indicated in figure 6. Increasing temperature up to 60° C caused an increase in median crystal size and in crystal purity. The correlation between crystal size and purity and temperature was probably due to the decreased rate of absorption of HCl gas into solution at higher temperature, which decreased local super saturation and led to the formation of fewer nuclei. The decrease in the number of nuclei provided an environment in which crystals grew to larger sizes. Temperatures above 80° C did not further increase crystal size or purity. Since the vapor pressure of HCl is very high above 80° C, not enough HCl was absorbed to reach 26 pct concentration in the mother liquor. Consequently, the yield of ACH decreased.
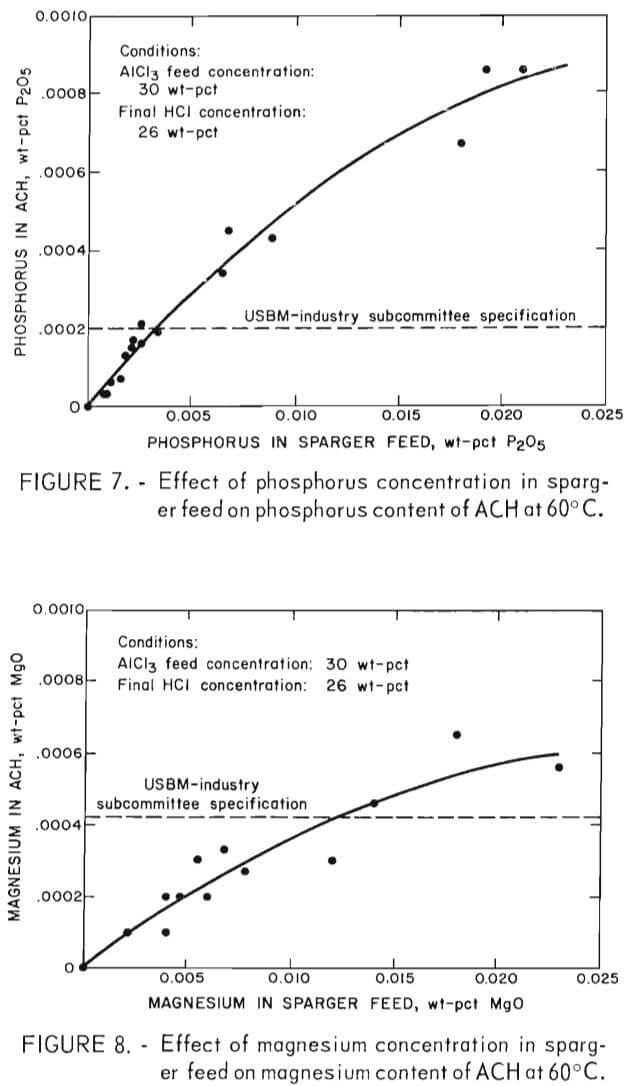
When ACH is decomposed to alumina, there is a 4.73- fold increase in concentration of impurities in the alumina. Therefore, to produce alumina that meets specifications of 0.001 wt- pct P2O5 and 0.002 wt-pct MgO, the P2O5 content of ACH cannot exceed 0.00021 wt-pct and the MgO content cannot exceed 0.00042 wt-pct. Because there is a direct relationship between the phosphorus and magnesium concentration in sparger feed and their concentration in ACH crystals, the maximum allowable concentration of phosphorus and magnesium in sparger feed can be obtained graphically. Figures 7 and 8 are enlargements of the low concentration portions of figures 4 and 5 and show the intersection of phosphorus and magnesium specifications, represented by dashed lines, with the 60° C curves. Intersection of the specifications on the 60° C curves shows that the maximum permissible concentrations in sparger feed are 0.003 wt-pct P2O5 and 0.01 wt-pct MgO, respectively.
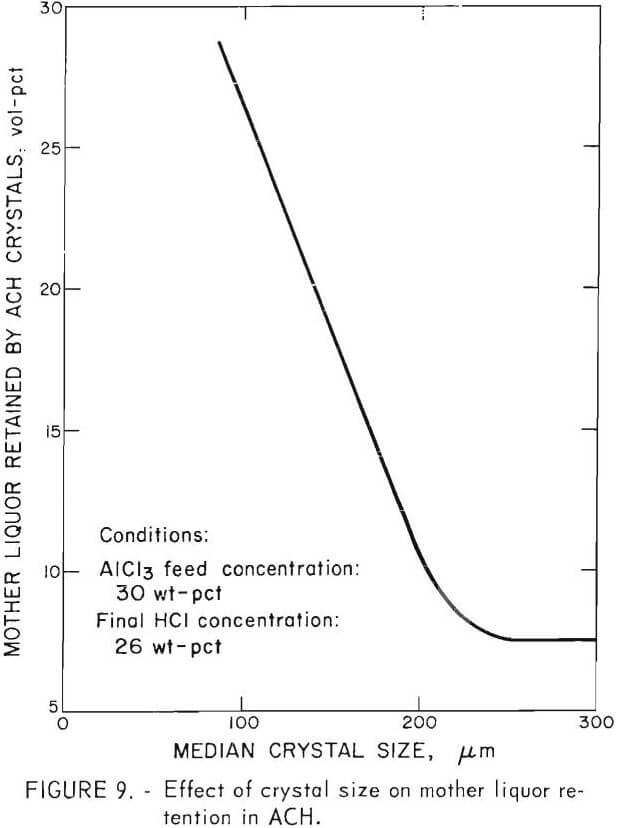
Crystal size affected washing efficiency, particularly for ACH crystallized at 20° C. At 20° C, a viscous mass of small crystals quickly formed and did not separate from the liquor, even after several minutes of settling time. The effect of crystal size on the amount of mother liquor retained in the ACH crystals after filtration is shown in figure 9. Crystals produced at temperatures of 40° C or greater (median size 235 to 328 µm) retained 6 to 9 vol-pct mother liquor and were easily washed. Crystals produced at 20° C (median size 66 to 106 µm) retained about 25 to 42 vol-pct mother liquor, and washing was very difficult. However, all crystals in this study were well washed to insure that washing was not a parameter in final crystal purity.
 Effect of Aluminum Chloride Concentration on ACH Crystal Purity
Effect of Aluminum Chloride Concentration on ACH Crystal Purity
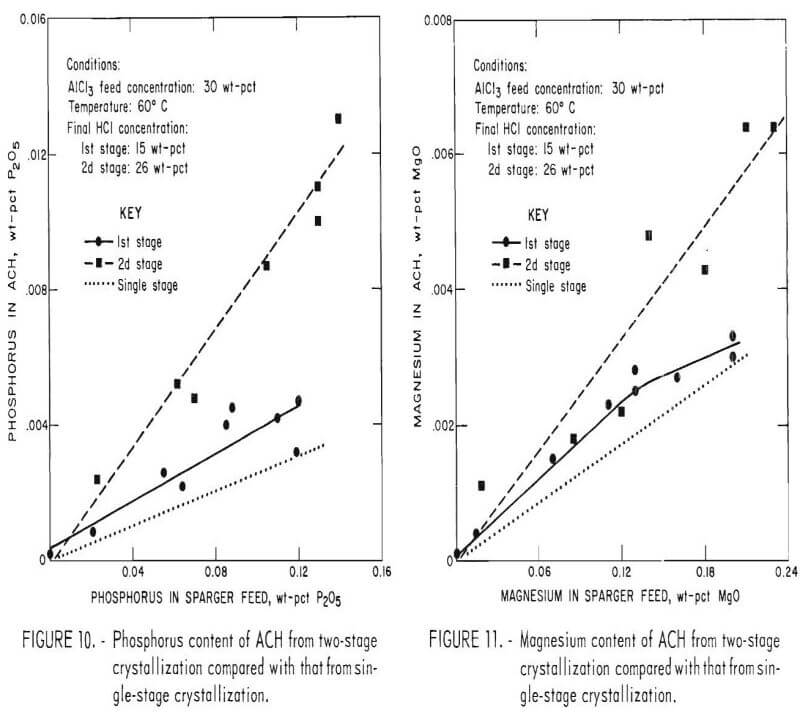
The majority of tests were performed by increasing the HCl concentration of the mother liquor to 26 wt-pct in a single stage. However, some crystallization tests were performed in two stages. In the two-stage tests, the ACH crystals were removed in two batches—the first batch when HCl concentration reached 15 wt-pct, and the second batch when the HCl concentration was 26 wt-pct. A series of two-stage crystallization tests was conducted at 60° C on 30 wt-pct-AlCl3 sparger feeds containing a wide range of phosphorus and magnesium. Figures 10 and 11 are plots of the effect of phosphorus and magnesium concentration in the sparger feed on the phosphorus and magnesium content of the ACH crystals, comparing two- stage crystallization with single-stage crystallization. The crystals produced in both the first and second stages are more contaminated than the crystals obtained in a single stage of sparging. This is very noticeable in the phosphorus curves but only slightly noticeable in the magnesium curves. The impurity content of the single-stage crystals should be the average of the impurity contents of the first- and second-stage crystals. An explanation for this observation may be that the major portion of the phosphorus is incorporated in the crystals during the early stage of crystal growth, and relatively little is incorporated during the subsequent, slower crystal growth stage. If the process is interrupted by removing all the crystals (at 15 wt-pct HCl), nucleation must occur again for the crystals produced during sparging from 15 to 26 wt-pct HCl. Both sets of crystals will contain more phosphorus than if crystal growth had proceeded without interruption in a single stage.
The second-stage ACH crystals contained significantly more phosphorus than either first-stage or single-stage ACH crystals. The most obvious difference in conditions was that second-stage crystallization began in a solution containing 15 pct AlCl3 and increased concentrations of impurities. The increase in impurity concentrations as sparging proceeded was noted in all batch sparging experiments because less than 1 pct of the impurities was removed during crystallization while solution weight decreased considerably due to AlCl3 removal. For example, the mother liquors from sparger feed solutions initially containing 0.12 wt-pct P2O5 contained 0.14 wt-pct P2O5. To determine if the AlCl3 concentration of sparger feed affected crystal purity, a series of tests was made with the phosphorus concentration kept constant at 0.12 wt-pct P2O5 and the AlCl3 concentration varied. Figure 12 shows that AlCl3 concentration is an important parameter and that AlCl3 concentration should be 30 pct at the beginning of crystallization. 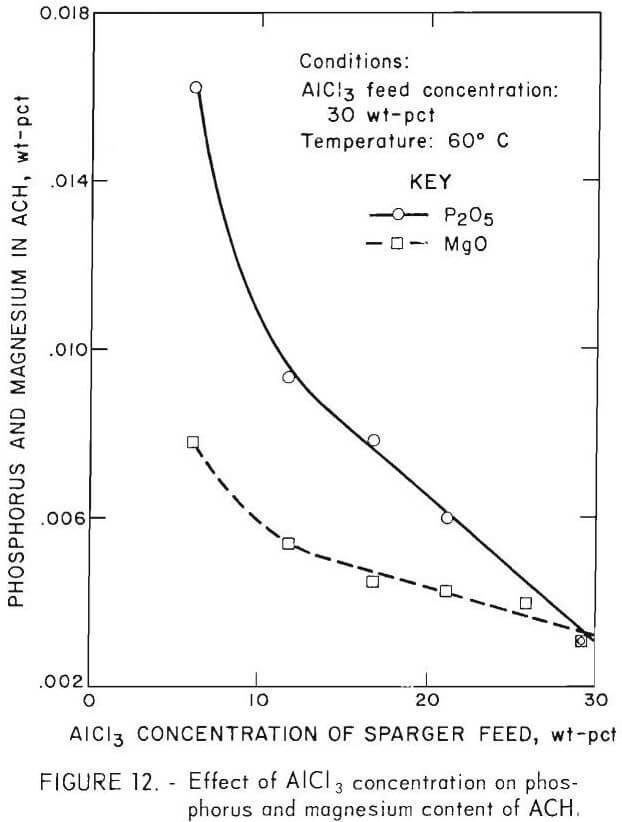 Aluminum chloride concentration and phosphorus incorporation during the early stage of crystallization are probably the main factors in determining the phosphorus content of the ACH. Since only a very small crystal yield can be obtained from a 5-pct-AlCl3 sparger feed, the crystals will be relatively highly contaminated because crystal growth stops soon after nucleation.
Aluminum chloride concentration and phosphorus incorporation during the early stage of crystallization are probably the main factors in determining the phosphorus content of the ACH. Since only a very small crystal yield can be obtained from a 5-pct-AlCl3 sparger feed, the crystals will be relatively highly contaminated because crystal growth stops soon after nucleation.
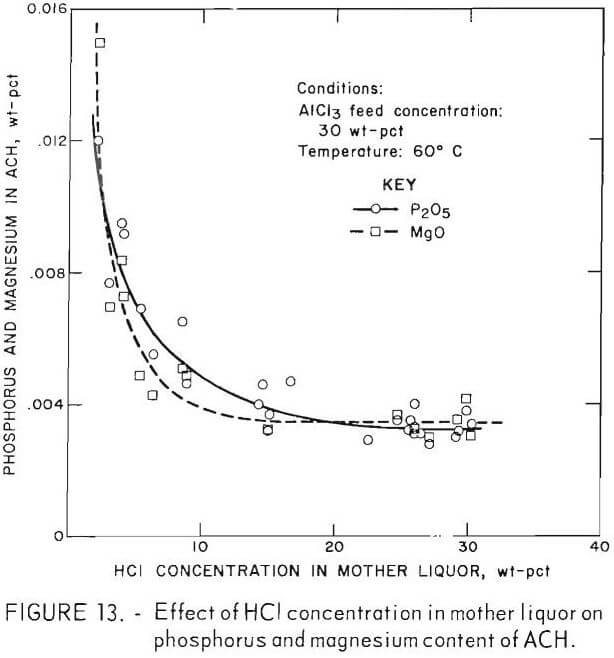
Other experiments were performed which indicated that major phosphorus and magnesium incorporation occurred during the early stage of crystal growth. A series of 30-pct-AlCl3 sparger feeds containing phosphorus and magnesium were sparged for periods ranging from 0.75 to 7.0 hours and analyzed. Figure 13 shows the phosphorus and magnesium content of the ACH as crystallization proceeds. The first crystals produced were highly contaminated, but as crystallization and crystal growth continued, the relative contamination decreased.
In another study a pure AlCl3 sparger feed containing no impurities was sparged, and as soon as crystallization was observed, phosphorus and magnesium were added to bring their concentrations to 0.12 wt-pct P2O5 and 0.22 wt-pct MgO. The ACH crystallized by sparging to 26 pct HCl contained only one-third of the phosphorus and one-half of the magnesium usually incorporated into crystals sparged from liquor of this composition. Therefore, seeding with pure ACH to provide clean nuclei for crystal growth may produce significantly cleaner crystals from contaminated liquors.
Conclusions
A batch bench-scale crystallizer was designed and operated to provide information on the HCl sparging crystallization of aluminum chloride hexahydrate from aluminum chloride liquors in order to optimize the crystallization operation.
A study of a single ACH crystallization step showed that Bureau of Mines industry specifications for purity were met for all contaminating elements except phosphorus and magnesium.
Aluminum chloride hexahydrate of cell-grade purity can only be crystallized from saturated aluminum chloride solutions containing less than 0.003 wt- pct P2O5 and 0.010 wt-pct MgO. Four important parameters in controlling the concentration of the phosphorus and magnesium in aluminum chloride hexahydrate crystals produced in a batch, bench-scale crystallizer were (1) aluminum chloride concentration in feed to sparging, (2) hydrogen chloride gas flow rate, (3) temperature, and (4) concentration of phosphorus and magnesium in the feed to sparging. Purity was enhanced by conditions that led to the formation of large crystals.
The chemical and physical forms of the phosphorus and magnesium impurities in ACH were not determined. However, the incorporation of these impurities into ACH was apparently greatest during the early stage of crystallization. The first crystals to form contained disproportionately large concentrations of phosphorus and magnesium, while much less phosphorus and magnesium were incorporated into the crystals in the later stages of crystallization. Therefore, it is very important to control nucleation and crystal growth.
