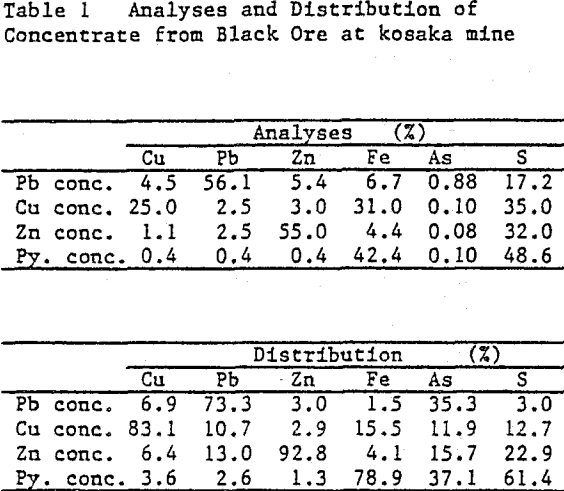
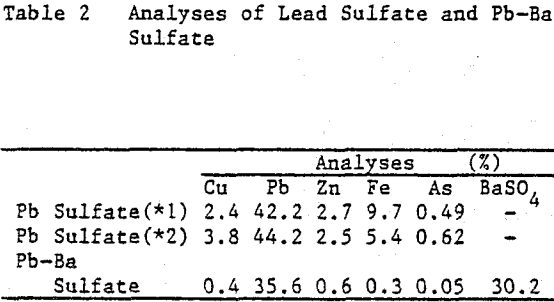
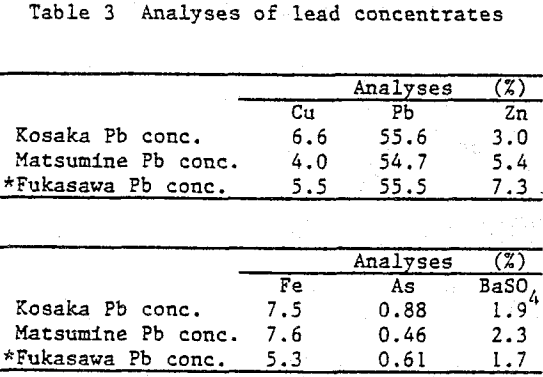
Lead concentrates from Kuroko (black ore) mines, which contain Cu 4 – 7%, Pb 55%, Zn 3 – 7%, As 0.5 – 0.9% are treated by hydrometallurgical process in order to smelt the product by electric furnace smelting together with other lead resources from Kuroko. The hydrometallurgical pretreatment involves sulfuric acid leaching at first stage, ferric leaching at second stage.
History
In 1967, Outokumpu flash smelting furnace started up to smelt copper concentrate from Kuroko, taking the place of blast furnace which operated for seventy years since 1897.
Matte and slag from the short rotary furnace was charged into the converter in molten state. By this process, it became possible to recover Cu, Pb, Bi and precious metals. However, Zn, Cd and As could not be recovered as before, and especially Cd and As in the waste water from the plant were not desirable with regard to environmental pollution control.
In April of 1979, it was decided to increase lead smelting capacity to 2,000 ton per month of electrolytic lead from 1,400 ton per month, treating 1,100 ton per month of lead concentrate as new resources.
The basic factors governing our decision were as follows:
- We have twenty year’s experience of electric furnace smelting for lead sulfate and three year’s experience for Pb-Ba sulfate.
- Our Pb % of lead concentrate is too low to apply mutual reaction smelting between PbS of the concentrate and PbSO4 of the lead sulfate.
- Also, Pb-Ba sulfate can not be sintered.
- Electric power cost in night time (from 10 PM to 8 AM and holiday) is discounted in Japan when power consumption ratio of night time to one day is 532 or over.
- The average refractory life of short rotary furnace was ten months. If we smelt Pb-Ba sulfate with this furnace, the life will be shortened further.
Laboratory Test
The purpose of laboratory test was to select the best process suitable for converting lead sulfide to lead sulfate. Basically, there are three types of hydrometallurgical, conversion processes.
Type I : Sulfuric acid leaching
PbS + H2SO4 = PbSO4 + H2S……………………………………………(1)
Type II : Ferric leaching
PbS + Fe2(SO4)3 = PbSO4 + 2FeSO4 + S°………………………………(2)
Type III : Oxidation leaching
PbS + 2O2 = PbSO4………………………………………………………(3)
In view of the results of these tests, it was found that conversion to lead sulfate was possible by both methods, but dissolution of copper, zinc and arsenic were realized only by ferric sulfate leaching. However, for ferric leaching, a large amount of ferric sulfate was needed.
Commercial Plant
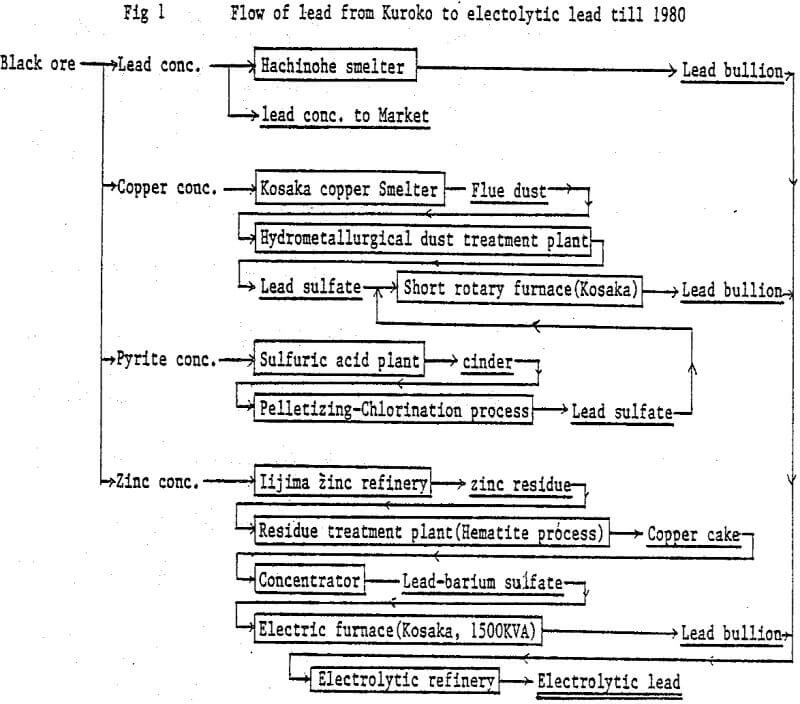
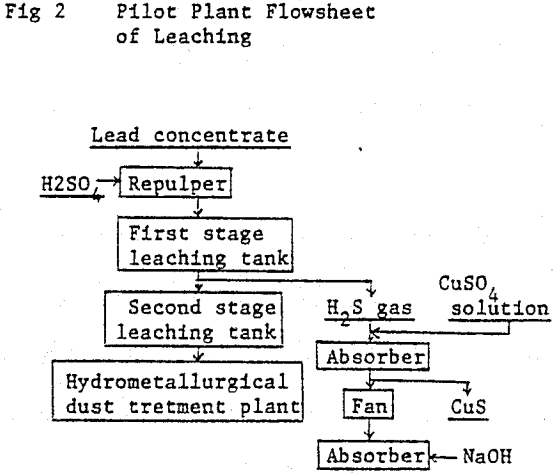
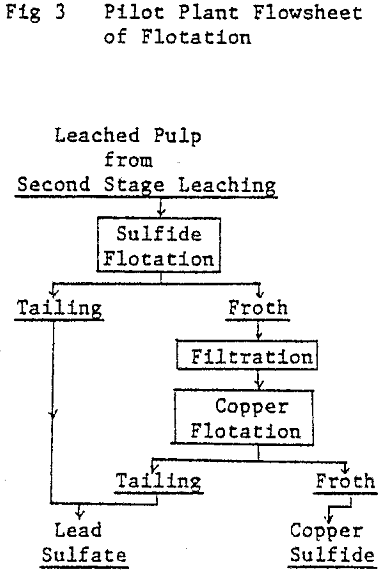
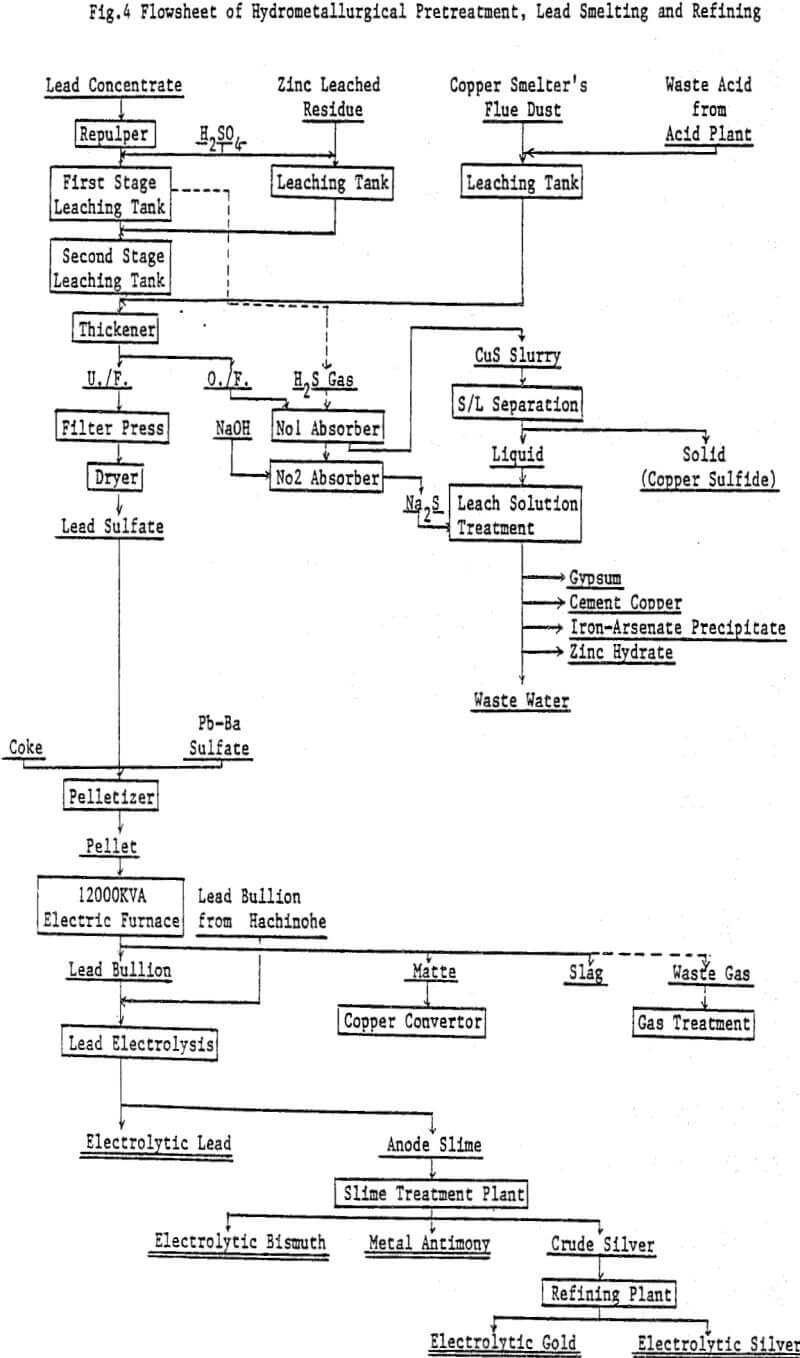
The commercial plant was designed to employ the two stage continuous leaching process and to have a treatment capacity of 50 tons of lead concentrate per day. The overall flowsheet, including hydrometallurgical dust treatment, lead smelting and refining.
It was decided that the new plant should be located adjacent to the existing hydrometallurgical dust treatment plant, taking account of the easiness of H2S gas controlling.
Flowsheet of commercial plant is as follows:
Repulp: Lead concentrate is slurried with water to 60% solids.
First stage leaching: Leaching tank is being kept at a temperature of 90°C and 90 g/l of sulfuric acid concentration. Lead concentrate slurry and sulfuric acid are separately charged into the leaching tank by respective pump, and the leached residue overflows to the next leaching tank.
Second stage leaching: The overflown slurry comes in contact with ferric sulfate solution which is produced by hot acid leaching the zinc leach residue in a separate leaching tank. Leached residue and solution go to a thickener. Leached residue is dewatered by a filter press, dried and sent to ore bin installed at the electric furnace plant.
H2S absorption: H2S gas liberated is drafted to a absorber, and contacts with copper sulfate solution which is obtained by leaching lead concentrate and copper smelter’s flue dust. A small amount of remaining H2S gas which cannot be absorbed in the absorber is completely fixed by NaOH solution as Na2S in the second absorber.