- To participate in the 911Metallurgist Forums, be sure to JOIN & LOGIN
- Use Add New Topic to ask a New Question/Discussion about Hydrometallurgy.
- OR Select a Topic that Interests you.
- Use Add Reply = to Reply/Participate in a Topic/Discussion (most frequent).
Using Add Reply allows you to Attach Images or PDF files and provide a more complete input. - Use Add Comment = to comment on someone else’s Reply in an already active Topic/Discussion.
GOLDIX (27 replies and 2 comments)
As I understand it, there is no cyanide concentration in GOLDIX 570; it is meant to be a cyanide-free chemical for dissolving gold.
There is zero cyanide, as it is not a cyanide dissolution system. If you have concerns re potential effluent problems with its use, contact GX & discuss solutions, as we understand there are many users world-wide.
There are at least five companies in China selling a "gold dressing agent". Information gleaned from their websites indicate all leach at a pH around 11-12. One company has a patent that states it is made from urea and ferricyanide. So, I believe they all contain alkaline thiourea and a small amount of cyanide. Another contributor on this forum reported that Goldix 570 has some cyanide present.
There may be one company with a gold leaching agent that is different since they claim it operates over a pH range of 3-11. More information from users of any of these so-called cyanide free agents would be appreciated.
It is time for a lesson in chemistry: the test for cyanide is not a reaction with silver nitrate or even the presence of ferricyanide. The test is adding a non-oxidizing, non-complexing acid, like hydrochloric acid, and measuring the amount of hydrogen cyanide gas that is released from the sample when it is strongly acidic.
Bob, how exactly do you carry out this without killing yourself considering HCN is what we always try to avoid by maintaining an alkali ph. Thank you. Adam
Analysis of free cyanide by titration with AgNO3 and potassium iodide as indicator is a well-known practice. http://is.gd/jJ3Bj5
You are correct but as stated that the reaction with silver nitrate was proof that free cyanide was present, which is absolutely wrong; I have been experimenting with the Chinese product for months and it has no cyanide content. Any good chemist knows that many chemicals react with silver nitrate besides free cyanide containing liquids, and in fact, the silver nitrate titration of which you speak cannot detect some cyanide containing chemicals such as the cyanides of gold and silver.
Yes, you cannot prove that there is cyanide in a liquid just adding AgNO3.
But you can analyze free cyanide by titration with AgNO3 and total cyanide as you have explained, provided that there is actually some cyanide, of course.
Let's say that after a qualitative analysis that shows evidence of cyanide, a quantitative analysis can give us free cyanide ( titration with AgNO3, for example ) and total cyanide by your method.
Correct, the silver nitrate titration for cyanide is the standard for determining the amount of free cyanide present, but this solution assay technique is only suitable for use in cyanide plants, not for use in general as a detection system for the presence of cyanide.
Another contributor a related discussion mentioned that the residual cyanide concentration after leaching with Goldix 570 was about 20ppm.
Who gave you that incorrect information? Titrating with silver nitrate, like you would for determining cyanide concentration in a cyanide plant DOES NOT PROVE THERE IS CYANIDE PRESENT. The silver nitrate titration technique is used for determining the concentration of many other chemicals besides cyanide.
The method by which cyanide was measured was not discussed. While you may have a valid point of view, all I did was try to answer the question being posed. The patent I mentioned (not held by Florrea who sells Goldix 570) makes it very likely that cyanide is used to formulate the leaching agent. I am assuming it is the same for about four companies.
I asked for more information from users of these products, I am posting the response about GOLDIX from the thread about alternative methods to mercury and cyanide.
We can now use Goldix 670 which is non-toxic solvent for gold. It is a substitute for cyanide. Our recent pilot results are recoveries of about the same with Cyanide but the consumption is slightly higher applied in ore that is quartzitic with low sulfides.
The toxicity is low. Compared to Cyanide in a standard CIL While the Cyanide treated CIL showed Cn at the end of the CIL of about 200 ppm before detoxification the equivalent Cn reading with Goldix 670 as solvent is only 21 ppm. This is a substitute that is now available in the market.
I have been experimenting with the Florrea product for several months. It operates in alkaline conditions, like cyanide, and concentration is determined with silver nitrate titration, like cyanide, but it does not contain any detectable amount of cyanide. Like cyanide, is dissolves both gold and silver, but unlike cyanide, it attacks and dissolves many metals including platinum, palladium and, unfortunately, even iron hence steel agitation tanks are out of the question.
How fast does the leaching occur in agitation tanks? Most companies selling dressing agents have touted its use with heap leaching. Hence, the leaching rate was slow.
Also, what percent dissolution is possible?
We are a large coin mint and refinery of gold and silver in California and seek a cyanide alternative for recovering precious metal values from low-grade material. The Florrea product requires oxygen to dissolve the metals, like cyanide does, hence we have been using air agitated glass cylinder in our research. Where I am currently employed, we are not interested and have not tried using the chemical for mineral heap leaching applications, but it seems quite fast in stripping precious metal platings off of substrates. Unfortunately, it also attacks the substrate if it is metallic.
The chemical I received requires alkaline conditions and oxygen just like cyanide does, but appears to be more aggressively than cyanide because it attacks and dissolves many other metals besides gold, silver and copper. However, I cannot make a direct comparison because I am employed in California where we have no access to cyanide due to State laws. I do not have the ability to quantitatively or qualitatively analyze the composition of this chemical, nor do I care of what or how it is manufactured; my interest is to discover a chemical that does the job I need for my employer in this State which does not permit new use of cyanide.
Goldix contains cyanide in a form in which it is only reactive with noble metals and has a small but not negligible toxicity (equal to caustic soda). That is why it is so compatible with existing CIP/CIL etc circuits. Method to analyze gold-CN complex concentration in solution works the same way with goldix.
Goldix doesn't like zinc cementation because it would remove the iron content of the product. It is well known that ferricyanide (oxidized ferrocyanide) enhances cyanidation.
Goldix is an anhydrous product (contains Na2O), it is obtained by fusion. It is mainly made of urea, ferrocyanide and some leaching enhancement product: NH4 which I suppose releases NH3 in alkaline media and help to dissolve silver, some deflocculating agent (CaCl2, sodium hexametamosphate). There are few Chinese patents with almost the same recipe.
Cyanide dissolves well palladium; you must cyanide it at pH 9/9.5. With platinum it is more difficult, but there are some compounds that enhance dissolution.
Well, I don't have access to a goldix sample but to know equivalent CN concentration in goldix, somebody might do this:
React know mass of goldix with strong acid (sulfuric or whatever) and heat (boil!)
Scrub the gas (HCN, take care) in a known sodium hydroxide solution
Dosage of CN in the sodium hydroxide solution with the standard method (AgNO3).
Has anyone conducted a WAD cyanide measurement on leach solution prepared with this reagent by distillation or gas membrane techniques? This would be almost conclusive as to the amount of "available" cyanide present as opposed to that complexed with iron.
Searching the web (Alibaba.com is a good place to look); i have found about six companies selling gold dressing or gold separation agents. They all appear to be based on the use of alkaline thiourea along with sodium ferricyanide, sodium sulfite, and/or sodium silicate. This is based on the research by Dr. Chai and some of his students Dr. Wang and Dr. Zheng. Patents were filed around their findings (CN1295356, CN1667140A) in 2005 and 2007. A private company discovered they could heat up urea along with ferricyanide and some other ingredients (probably the ones previously listed) to create the Golden Cicada Dressing Agent. The patents for this process (CN2011002086, WO2012079283) are available. Several other companies also sell this agent or similar agents (under different names). More recently another company has patented almost the same process (CN103276206) in 2013. All of these companies add sodium ferricyanide as an oxidizing agent (in much lower concentrations of cyanide than used with normal cyanide leaching). A new patent of interest that uses thrioureas, hyrdogen peroxide, EDTA and some polyphosphates lacks cyanide (CN102121067B). I have not yet determined whether the last formulation is being sold. I am curious by the comments by that Goldix 570 appears to be very reactive and leaches a variety of heavy metals. I should note that the research by Wang (2005, 2006) found that alkaline thiourea is selective in only leaching gold (and possibly silver), unlike cyanide (at high pH) or acidic thiourea which does leach heavy metals in addition to gold. A recently commercial "gold separation product" called DST is capable of leaching gold, silver, and some copper. I am interested in these "agents" because of the selectivity for gold. More information is required to determine whether some agents being sold are more selective than other company products.
Goldix is a thiocyanate https://www.911metallurgist.com/blog/iron-in-cyanidation-ferrocyanide-compounds
The State of Montana has shared with me info on potential use of a “cyanide alternative” which you believe to be thiocyanate or a chemical called “Rhodanide.” DEQ has experience with thiocyanate at the Mine. The cyanide solution in the heap evolved into a 1300 ppm thiocyanate solution which was not only resistant to the traditional cyanide “kill” circuit in the mine closure plan, but also proved to be an extremely toxic herbicide to every plant in the land application area. DEQ had to build an entirely new million dollar biotreatment plant (not anticipated in the closure budget) to deal with the problem. Furthermore, thiocyanate is a sulfur-cyanide compound, and therefore is a “cyanide ore-processing reagent” as defined in 82-4-303 (5) MCA: “cyanide or a cyanide compound used as a reagent used in leaching operations.” 82-4-390 (1) MCA further adds that “Open-pit mining for gold or silver using heap leaching or vat leaching with cyanide ore-processing reagents is prohibited.
Use the Social Share Bar on the Left. Tell everyone you can about https://www.911metallurgist.com/metallurgy/ It's FREE & GOOD.
Laboratory tests conducted in Canada using a cyanide-free leach agent produced interesting results when compared to cyanide itself.
The sample tested was of refractory nature with 48% sulfur (high pyrite) VS your oxide material being 3% sulfur.
Gold leached at around 50% using either chemicals. Silver leached at 25% using the cyanide-free leach agent VS 75% using standard cyanide.
Below is a list of benefits listed be the manufacturer:
- Consumption: Same as Cyanide on certain type of ore
- Leaching rate/time: Similar to cyanide
- Less affected by detrimental metals, like As, Sb, Sulfide.
- Same method of application with Cyanide
- pH:10 to 12 using lime as regulator
- Lower cost of risk management, transportation and inventory.
- Less cost of tailings disposal. Detoxification not needed.
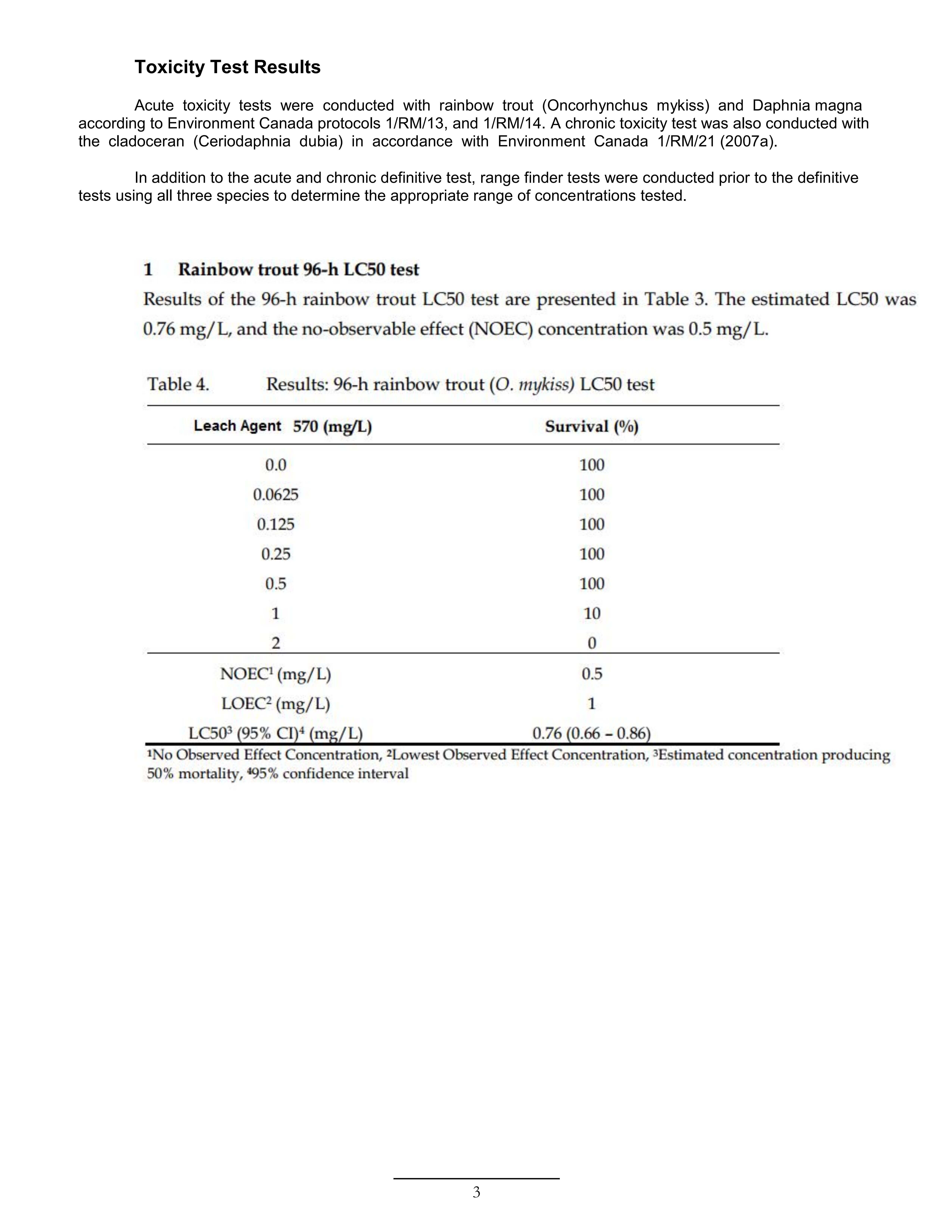
Toxicity Test Results
Acute toxicity tests were conducted with rainbow trout (Oncorhynchus mykiss) and Daphnia magna according to Environment Canada protocols 1/RM/13, and 1/RM/14. A chronic toxicity test was also conducted with the cladoceran (Ceriodaphnia dubia) in accordance with Environment Canada 1/RM/21 (2007a).
In addition to the acute and chronic definitive test, range finder tests were conducted prior to the definitive tests using all three species to determine the appropriate range of concentrations tested.
Use the Social Share Bar on the Left. Tell everyone you can about https://www.911metallurgist.com/metallurgy/ It's FREE & GOOD.
Use the Social Share Bar on the Left. Tell everyone you can about https://www.911metallurgist.com/metallurgy/ It's FREE & GOOD.

Use the Social Share Bar on the Left. Tell everyone you can about https://www.911metallurgist.com/metallurgy/ It's FREE & GOOD.
Use the Social Share Bar on the Left. Tell everyone you can about https://www.911metallurgist.com/metallurgy/ It's FREE & GOOD.
I understood that SC 570 Gold is one of the varieties Goldix 570
I am interested in the method of its determination in solution. The procedure is recommended to determine how well and cyanide, but I did not get.
I of the reagent preparing a solid powder of 10% solution, then titrated in the presence of KJ its 5% AgNO3 solution dropwise. The solution does not change color. Where is the mistake ?
Hi Mikhail,
Best contact Florrea (Goldix):
Yang Zhiyong
President
Mobile: + 86.136.0982.1616
Tel: +86.24.3151.5191
Fax: +86.24.3151.3277
Email: yang.zhiyong@florrea.com
Skype: yang.zhiyong
MSN: yang_zhiyong1616@hotmail.com
or Vita (gold dressing agent)
QINGDAO AIWEISHENG Cgold dressing agentHEMICAL CO.,LTD
Tel:+86-0532-89267645
Mobile:+86-13472167952
Whatsapp/Viber:+8613472167952
Skype: vita198911
Email: vita@qdawschem.com
Vita once told me: While doing experiments with calcium oxide CaO or hydroxide NaOH to adjust pH value, during the process of stirring every half hour measure pH, once pH stable at 10 to 11 began to add in gold dressing agent, calculate based on every kilograms of ore per gram of gold use 0.5g gold dressing agent, calculate the total apply amount of gold dressing agent according to the total ore amount and grade (total apply gold dressing agent amount = total ore amount(kg) x grade(g.kg) x gold dressing agent(0.5g/g.kg), get the total gold dressing agent amount divided by 4=1/4, at the beginning put 1/2, every 4 hours put 1/4, 12 hours after wash the tailings until the pH value is neutral to test the result, during the process of stirring using activated carbon adsorb the dissolved gold, you can add a small amount of hydrogen peroxide H2O2.
Be sure to review the 3 PDFs at the bottom of https://www.911metallurgist.com/blog/goldix
Use the Social Share Bar on the Left. Tell everyone you can about https://www.911metallurgist.com/metallurgy/ It's FREE & GOOD.
Please join and login to participate and leave a comment.

Does anyone know the actual free cyanide concentration in GOLDIX 567 OR 570 and where do I find GOLDIX for sale?