- To participate in the 911Metallurgist Forums, be sure to JOIN & LOGIN
- Use Add New Topic to ask a New Question/Discussion about Hydrometallurgy.
- OR Select a Topic that Interests you.
- Use Add Reply = to Reply/Participate in a Topic/Discussion (most frequent).
Using Add Reply allows you to Attach Images or PDF files and provide a more complete input. - Use Add Comment = to comment on someone else’s Reply in an already active Topic/Discussion.
Lead nitrate & peroxide addition in leaching (2 replies and 1 comment)
Look here:
https://www.911metallurgist.com/hydrometallurgy/effect-of-lead-nitrate-on-gold-leach-kinetics/
Lead nitrate sometimes works and sometimes does not, it usually depends if there are sulfides present. There are a lot of theories as to why it works but typically people just try it in the lab and see. Dosages in the range of 0.05 to 0.5 kg/t are common.
As for peroxide, it can serve two purposes, oxidize the sulfide surface to reduce cyanide consumption or as an oxygen booster. Often it is added in a pre-aeration step to augment air to oxidize surfaces, it can also be added directly to the CN leach but it can oxidize CN as well so care on dosage has to be taken. See this link
Regards
Todd Harvey - Global Resource Engineering http://www.global-resource-eng.com
As stated by Todd and the original article titled "Developments in gold leaching using hydrogen peroxide: by Loroesch, J.; Griffiths, A.; Knorre, H. in Society for Mining, Metallurgy & Exploration
Hydrogen peroxide is known to the mining industry as a powerful oxidant, able to attack and to destroy cyanide, the most important leaching reagent.
CN + H2O2 ⇒ OCN + H2O
The most recent development, however, is the use of H2O2 as an oxidant in the cyanidation of precious metals.
This application may appear to be a contradiction to the above mentioned detoxification process. This can, indeed, be the case if certain conditions are not considered carefully. A new process to enable the application of H2O2 in this field without having any detrimental effect on the cyanide.
The incentive to seek an oxygen supplier like H2O2 instead of air was the fact that one of the most important conditions for the fast dissolution of gold is the presence of physically dissolved oxygen in the pulp.
2 Au + 4CN- + ½ O2 + H2O ⇒ 2 [Au(CN)2]- + 2 OH
Complete gold recovery within a reasonable leaching time may only be achieved if the pulp is saturated with dissolved oxygen. Gold losses in leach operations mainly occur due to the following reasons:
In some cases, the saturation of oxygen in the pulp cannot be achieved by aeration due to the difficulty to transfer oxygen from the gaseous into the liquid phase. This happens, for example, in pulps with a high viscosity. In such cases, even violent aeration only results in a low concentration of dissolved oxygen in the pulp.
In any case, the starting phase of leaching requires the highest amount of oxygen. Therefore, it is especially important to raise the dissolved oxygen level to saturation in the starting phase.
The intention of the peroxide assisted leach process PAL is to introduce oxygen in a liquid form. As a result of intensive research work, it could be shown that hydrogen peroxide can be used as a source of oxygen in the leaching of gold ores. To avoid side reactions, the following conditions must be applied:
- Hydrogen peroxide must be diluted before it is added to the pulp.
- The dosage of H2O2, must be properly regulated based on the oxygen level of the pulp.
- An important part of the continuous O2-control is a special oxygen electrode, which resists the extremely abrasive action of the pulp.
To compare the efficiency of H2O2 with common oxidants used in cyanidation, the authors have investigated the oxygen profiles in the pulp using H2O2 pure oxygen, and violent aeration with compressed air.
During testing, the final O2-level of 12 ppm was reached already after 14 minutes using H2O2 while a level of 8 ppm was obtained only after three hours with pure oxygen. With violent aeration, a maximum O2-level of only 4.3 ppm was obtained after one hour. This illustrates that the active oxygen in H2O2 can be used much more efficiently. Even the addition of six times the amount of active oxygen as pure oxygen or more than 800 times the amount of oxygen in compressed air did not allow the achievement of the same performance as hydrogen peroxide. It is also easy to exceed the oxygen saturation limit of the pulp (about 9 ppm at sea level) by more than 100% using H2O2 as an oxygen supplier.
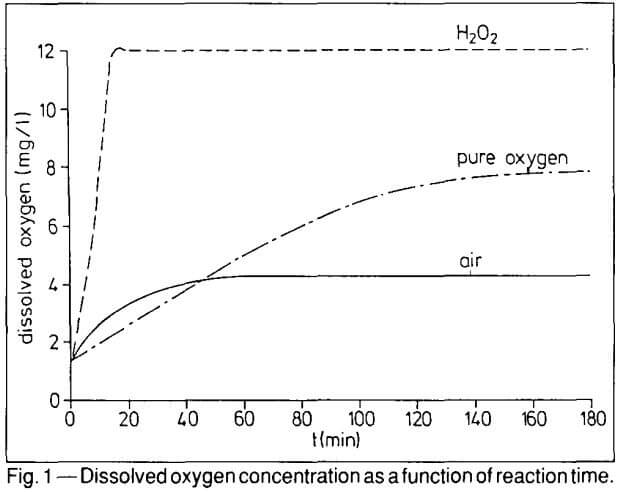
The dissolution rate of precious metals in the cyanidation process is directly related to the concentration of dissolved oxygen in the pulp.
Please join and login to participate and leave a comment.

Hello everyone, in any gold or silver leach plants: