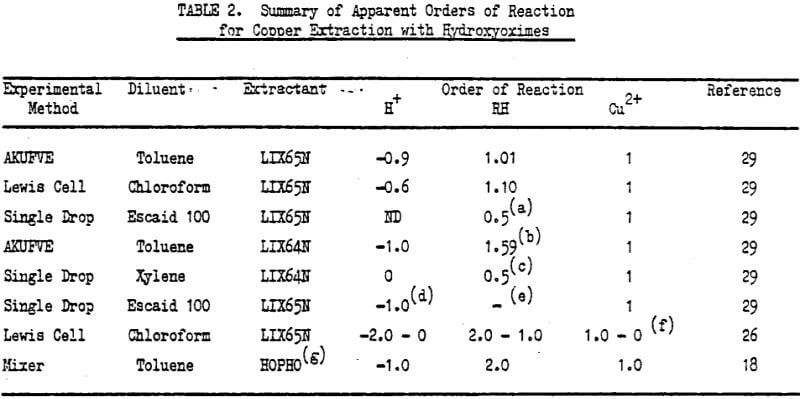
The kinetics of solvent extraction with hydroxyoximes are complex, involving mass transfer with chemical reaction in a heterogeneous system. One is therefore faced with a wide spectrum of possible behaviour within certain well defined boundary conditions. The extraction rate could be diffusion-controlled under all conditions and would thus depend on the interfacial area, and concentration of the slow diffusing species.
When the extraction rate is chemically controlled it is important to ascertain the location of the rate—controlling chemical reaction or reactions i.e. within a bulk phase or at the interface. For a bulk phase rate-controlling chemical reaction the important parameters will be solubility of reactive species, their distribution coefficients (which will vary with diluent choice and ionic strength of the aqueous phase), ionisation constants, if appropriate, and phase volume. For an interfacial chemical reaction under diffusion controlled kinetics the composition of the interface will correspond to the concentration of species as given by the equilibrium expression for the interfacial reaction. When interfacial chemical reactions are rate controlling then the important parameters are interfacial activity of reacting species, and molecular geometry with respect to preferential molecular orientation at the interface. Under such rate controlling conditions the composition of the interface will be that of reactants only. Thus physical chemical data for the extractants, relating both to bulk phases and the interface, will be of use in interpretation of the kinetic data.
Physical Properties of Hydroxyoximes
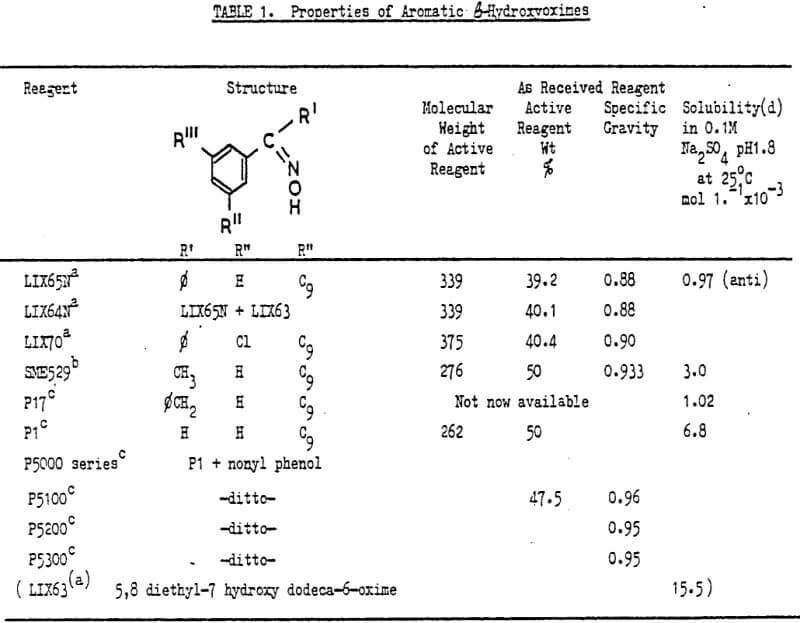
The properties of commercially available aromatic β hydroxyoximes are shown in Table 1. Extensive studies by Ashbrook have shown the active reagent to be the anti-isomer and spectra, structure and proton ligand stability constants have been obtained. Gas chromatography has been shown to be particularly effective for separating aliphatic and aromatic hydroxyoximes ie LIX63 from LIX65N while anti— and syn-isomers can be separated by high pressure liquid chromatography or thin layer chromatography. Thin layer chromatography of the copper complex can be used to purify the oximes. The use of pure compounds is of high importance when obtaining physicochemical and kinetic data which are sensitive to impurities.
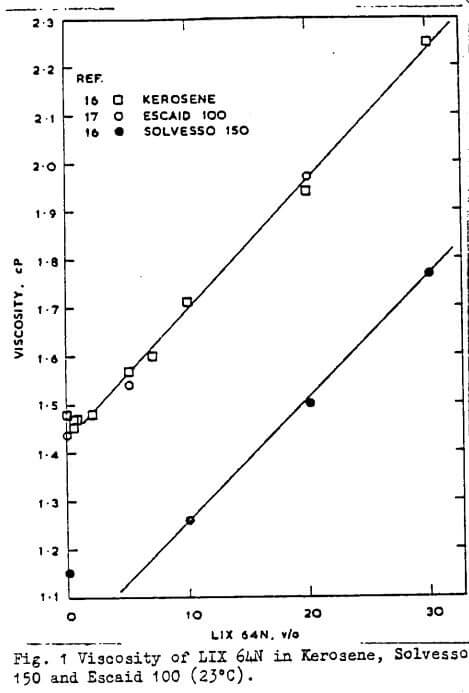
The degree of aggregation of several hydroxyoximes has been determined recently by Hanson et al for dry heptane at 28°C. The degree of aggregation decreases in the series LIX65N>P17>SME529>P50, and the addition of nonyl phenol caused a decrease in aggregation as did an increase in temperature and/or increasing aromatic character of the diluent. This diluent effect is in agreement with the findings of Dalton et al and Hummelstedt. Densities, viscosities and estimated diffusivities have been reported by Hughes et al who find that viscosities do not change dramatically as the concentration of extractant is increased to 30 v/o in various diluents.
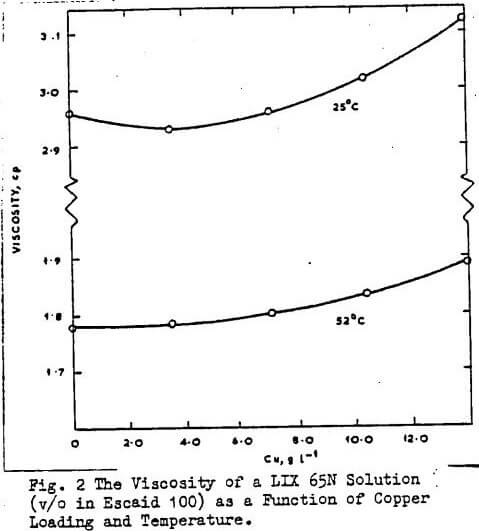
Furthermore, there is no dramatic increase in the organic phase viscosity as copper is loaded into the organic phase, unlike the behaviour of carboxylic- and dialkyl phosphoric acids, on metal loading. Results from other workers agree with these findings as shown in Figs 1 and 2. The diffusivity of LIX64N is higher in aliphatic than in aromatic diluents and the order of diffusivity in Escaid 100 was found to be LIX63>SME529>LIX65N.
Solubilities and partition coefficients for hydroxyoximes have also been reported. The solubility of purified extractants in an aqueous phase of pH value 1.78, as shown in Table 1, increase in the order LIX65N anti < SME529<P50<LIX63. LIX63 is reported as being some fifteen times as soluble as the anti-isomer of LIX65N while the copper (LIX65N)2 species is nearly ten times less soluble than LIX65N. The solubility of P50 increases slowly with pH until around pH10 when it increases dramatically. The solubility of P50 decreases with increasing temperature. Only the partition coefficient of P50 (10 4) has been successfully measured, interference by low molecular weight material in the samples or low aqueous solubilities have hindered measurements on other hydroxyoximes.
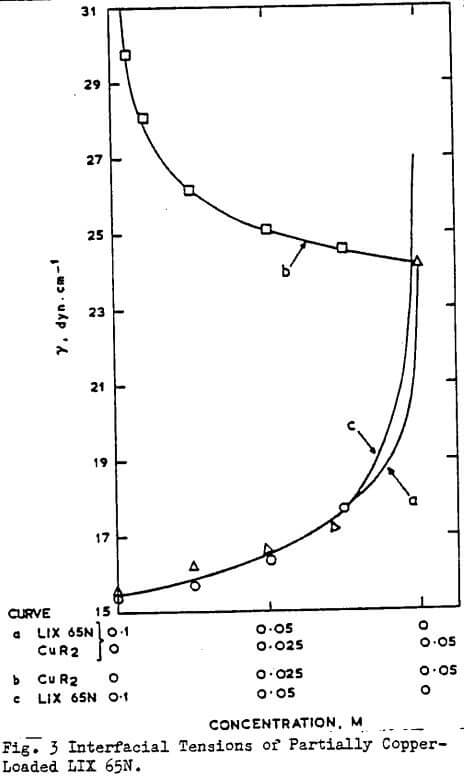
The interfacial tension of α- and β-hydroxyoximes and their copper derivatives have been measured by several workers. Great care is necessary in the interpretation of some of these data as the commercial reagents often contain other surface active compounds. Thus the calculation of interfacial area per molecule as carried out by some workers and attempts to correlate these with configuration of the molecule at the interface may be open to doubt.
As expected from the very weakly acidic nature of these extractants, no variation of interfacial tension with pH is found below pH9 and above pH 1.5 and the results obtained by Al-Diwan et al on commercial samples which are at variance with these findings are most probably associated with impurities in the system. The variation in interfacial pressure of 20 v/0 solution of LIX64N in various diluents has been reported, together with equilibrium and kinetic effects. The nature of the interfacial species in hydroxyoxime systems remains to be determined. Some workers have invoked the existence of dimers of the aromatic β-hydroxyoximes at the interface but it is more likely, in the author’s view, that considerable dissociation of the extractant aggregates occurs at the interface due to competitive hydrogen bonding with water molecules.
 |
 |
 |
 |
