Table of Contents
To meet these uranium demands, larger, lower grade, and refractory ore bodies will need to be exploited. The utilization of these ores will require the development of new and improved processing technology. In its program of Advancing Minerals Technology, the Bureau of Mines is investigating innovative methods for recovering minerals and metals from low-grade and refractory ores to maximize efficiency. This Bureau of Mines report describes methods for improving the extraction of uranium from ores partially refractory to conventional processing.
Most major domestic uranium ore deposits were formed when uranium in the soluble +6 oxidation state migrated through sandstone aquifers until the solution encountered a region containing sufficient reductant to reduce the uranium oxidation state to the considerably less soluble +4 state. Organic matter or H2S derived from the bacterial attack of sulfur-bearing components of this material often acted as the reductant. Consequently, many deposits of this type contain organic carbonaceous matter and sulfide minerals such as pyrite, chalcopyrite, and galena. In some instances, the uranium was encapsulated by the sulfide mineral or formed organometallic compounds of indefinite composition. Conventional leach processes are incapable of extracting the uranium from mineralization of this type.
Two basic processes are in use today to recover uranium from sandstone ores. The most common procedure employs dilute sulfuric acid leaching under oxidizing conditions to solubilize the uranium. Recovery of “yellow cake” a crude uranium oxide concentrate, is achieved in most cases after liquid- solid separation and purification by ion exchange or solvent extraction. This step consists of neutralizing the pregnant eluant with a suitable base such as NaOH, Mg(OH)2, or NH4OH, followed by thickening, filtration, and drying.
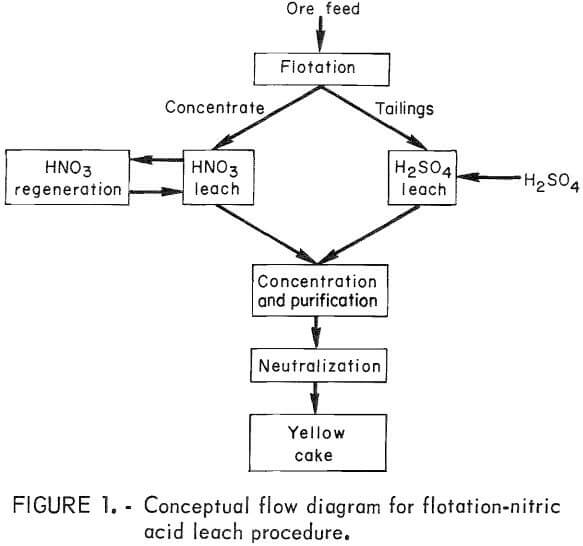
Flotation-Nitric Acid Leach Procedure
The other major process for uranium extraction is pressure leaching near 110° C with a solution consisting of Na2CO3 and NaHCO3 under oxygen pressure. It is most commonly employed when the ore contains gangue constituents that consume in excess of 150 to 200 pounds of H2SO4 per ton of ore. After leaching and liquid-solid separation, yellow cake is precipitated by raising the pH to near 12 with NaOH.
Two stronger oxidizing leach systems have been employed for more refractory ore types. The first is sulfuric acid-oxygen pressure leaching at 130° to 160° C. The second employs roasting near 600° C, followed by acid or carbonate leaching. However, incomplete metal extraction and high operating costs have combined to limit acceptance of these procedures.
Uranium ores that contain refractory minerals also have been treated by standard mineral beneficiation procedures into fractions that are amenable or nonamenable to conventional processing. The nonamenable portions have been subjected to roasting or pressure leaching before being incorporated into the amenable processing stream.
This report describes a procedure (fig. 1) consisting of froth flotation, followed by nitric acid leaching of the concentrate, and sulfuric acid leaching of the float tailings. This procedure was effective for increasing uranium extraction from an ore containing carbonaceous and pyritic refractory uranium mineralization.
Procedure and Apparatus
 The ore feed used in the investigation was obtained from a major uraniferous sandstone deposit. The chemical analysis of the H2SO4 compound and elements of primary interest was as follows, in percent: 0.25 U3O8, 1.40 Fe, 0.23 organic , 0.30 inorganic C, and 0.12 sulfide S. Quartz and feldspar were the major gangue minerals in this ore. Elements of minor interest contained in the ore were, in percent, 0.003 Cu , 0.004 Pb, and 0.05 V.
The ore feed used in the investigation was obtained from a major uraniferous sandstone deposit. The chemical analysis of the H2SO4 compound and elements of primary interest was as follows, in percent: 0.25 U3O8, 1.40 Fe, 0.23 organic , 0.30 inorganic C, and 0.12 sulfide S. Quartz and feldspar were the major gangue minerals in this ore. Elements of minor interest contained in the ore were, in percent, 0.003 Cu , 0.004 Pb, and 0.05 V.
Ore preparation entailed screening and grinding operations, in which the ore was first dried and then stage pulverized to 100 pct minus 35 mesh.
Conventional sulfuric acid leach experiments were conducted by adding 50 pounds of H2SO4 and 3 pounds of NaClO3 oxidant per ton of ore to a 55-pct- solids slurry of ore and water. This produced a final solution pH and oxidation potential of 1.5 and -500 mv, respectively, after 24 hours of agitation leaching. Liquid-solid separation was accomplished by filtering, repulping in water, refiltering, and then washing the filter cake on a filter funnel.
Flotation experiments were conducted in a 500-gram-capacity laboratory machine of the subaeration type. The ore feed in all experiments was conditioned at 1,800 rev/min after adding the reagents . A collection time of 5 min was then found to be sufficient.
Nitric acid leaching experiments were conducted in a standard laboratory 2-liter titanium autoclave equipped with a lifting turbine stirrer, solution sampling tube, gas vent, and solution injection port. Experiments were run at 50 pct pulp density for 1 to 4 hours. A pressure of 50 psig was maintained by manually venting off gases when the pressure reached this value. Temperature was also manually controlled, since electronic controllers allowed excessive temperature overshoot at the beginning of the exothermic reaction stage of each experiment.
Ion-exchange experiments were conducted in a burret containing a 10-ml volume of resin. Forty bed volumes of the leach solution were fed through the column of res in at rates between 0.75 and 1.2 ml/min (0.56 to 0.90 gal/min ft² resin), and effluent samples were collected every five bed volumes and
analyzed for uranium and iron. Uranium was eluted from the resin with a solution of 1M Na2CO3 as the stripping solution. Other stripping solutions such as acid chloride, nitrate, or sulfate systems could have been employed also.
Results and Discussion
Conventional sulfuric acid leaching extracted only 85 pct of the uranium from the ore. Electron microprobe analysis and heavy media separation experiments on the leached ore showed that the unleached uranium mineralization was encapsulated within sulfide grains and associated with unidentifiable carbon compounds. Because these types of minerals are often easily recovered by flotation, the experimental approach for concentrating the residual uranium was to float the sulfidic and carbonaceous constituents from the ore.
Flotation
Flotation experiments were conducted with conventional sulfide and coal collectors. The carbonaceous-sulfidic concentrates were then acid leached to remove leachable uranium prior to being analyzed for uranium. This procedure made it possible to determine the amount of refractory uranium recovered from the ore by the flotation technique. The highest recoveries were obtained by flotation at a pH of 4.5 with 0.80 pound of potassium ethyl xanthate collector and 0.05 pound of polypropylene glycol methyl ether frother per ton of ore at 20 pct solids. Under these conditions, 95 pct of the refractory uranium was recovered in the concentrate, and the concentrate-to-ore ratio was 1:80. The extremely high collector requirement was necessary to float the carbonaceous matter. Sulfides floated very well with as little as 0.01 pound of collector per ton of ore.
Nitric Acid Leaching
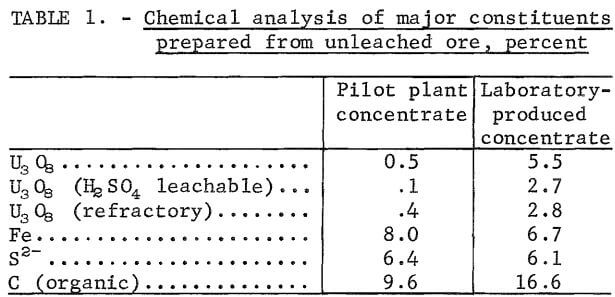
The large concentration ratio, when floating a concentrate, precluded production of sufficient quantities of concentrate to support laboratory leaching studies. But 145 pounds of a similar flotation concentrate that had been prepared from acid leach residue from the same ore deposit in commercial pilot plant operations was available. Comparison of the major constituents of interest contained in the two concentrates (table 1) shows that the pilot plant concentrate was considerably lower in grade in uranium and carbon and slightly higher in iron and sulfur content than the laboratory-produced concentrate, but adequate correlation of leach reagent requirements between pilot plant and laboratory-produced concentrates was found. This correlation will be presented later in the report. Spectrographic analysis showed that both concentrates contained major amounts of Si, 3 pct Al, 0.3 pct Pb, 0.1 pct V, and minor amounts of Ca, Na, Ti, Cu, and Ni.
Because the flotation concentrates contained minerals that were refractory to conventional acid leaching, several novel leaching techniques were evaluated for their efficiency in extracting the uranium. Procedures tested included chlorine-oxygen leaching, electrooxidation, and anodic oxidation. Chlorine-oxygen leaching extracted 63 pct of the uranium at a chlorine consumption of 46 lb/ton of ore and 79 pct at 164 lb/ton of ore. These experiments were conducted by reacting chlorine with an agitated concentrate-water slurry at 25° C for 1 hour. Oxygen then was administered, and the system was maintained at 100° C and 100 psig O2 for 24 hours.
Electrooxidation treatment extracted 50 pct of the uranium at an energy consumption of 510 kwhr/ton of concentrate. Forty-five pounds of NaOH per ton of concentrate was also required during electrolysis to maintain the pH near 8.0 because of acid-forming reactions.
Anodic oxidation was found to be a low extraction-high energy consuming technique.
Results from the preceding experiments indicated that approximately 80 pct of the uranium could be extracted from the concentrate by leaching, but that oxidant requirements would be high. Therefore, to reduce oxidant consumption, a system was sought whereby the oxidant could be regenerated. Nitric acid leaching appeared to be likely because its major reactions with pyrite and carbon generate nitrous offgases that are easily converted into HNO3 according to the following reactions:
2FeS2 + 10HNO3 → Fe2(SO4)3 + H2SO4 + 10NO + 4H2O…………………………(1)
C + 2HNO3 → CO2 + NO + NO2 + H2O…………………………..(2)
2NO + O2 → 2NO2,……………………………(3)
3NO2 + H2O → 2HNO3 + NO………………………………..(4)
The last two reactions proceed rapidly at 100° to 150° C with enthalpy of reaction values , H298° K , of -27.3 and -32.4 kcal/mole of product, respectively. After nitric acid attack on the pyrite and carbonaceous matter, the uranium would be leached with the sulfuric acid formed in equation 1 according to
UO2 2+ + xS04 2- → UO2(SO4)x (2x-2)-………………………………………..(5)
where x = 2 or 3.
A preliminary experiment employing HNO3 to leach the flotation concentrate in an open beaker indicated that nitric acid leaching was possible. The vigorous reaction raised the temperature from 80° to 95° C with the evolution of copious quantities of nitric oxide. A total of 75 pct of the uranium was extracted in 1 hour with 1,000 pounds of HNO3 per ton of concentrate. All subsequent experiments were conducted in the 2-liter titanium autoclave.
Effect of Amount of HNO3
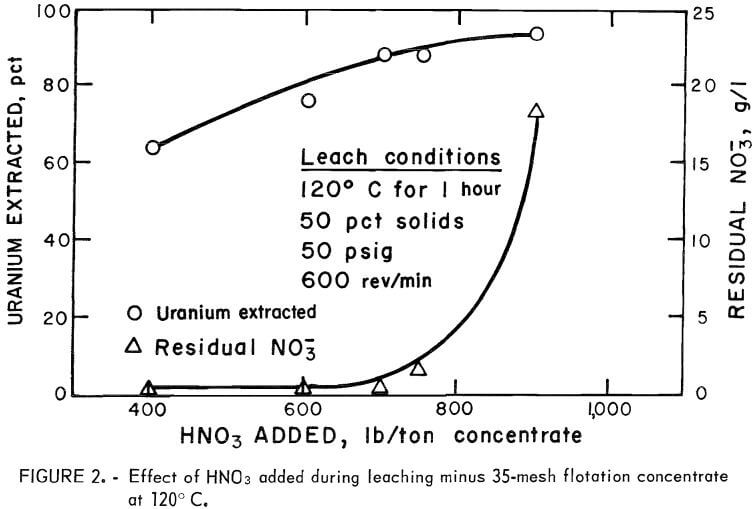
Experiments to determine the amount of nitric acid required for efficient leaching were conducted in the following manner. A charge consisting of 200 grams of flotation concentrate was mixed with 200 ml of water and sealed within the autoclave. The reactor was heated to 120° C, and 15-ml aliquots of HNO3 were added over a 15-min period. After acid addition, the temperature was maintained for 1 hour. Offgases were vented to control the temperature during the exothermic stage of the reaction and to prevent pressure buildup within the reactor. Results of these experiments (fig. 2) indicated that 700 pounds of HNO3 would be required per ton of concentrate for high extraction and the lowest practical residual nitrate level in the pregnant solution.
Effect of Particle Size
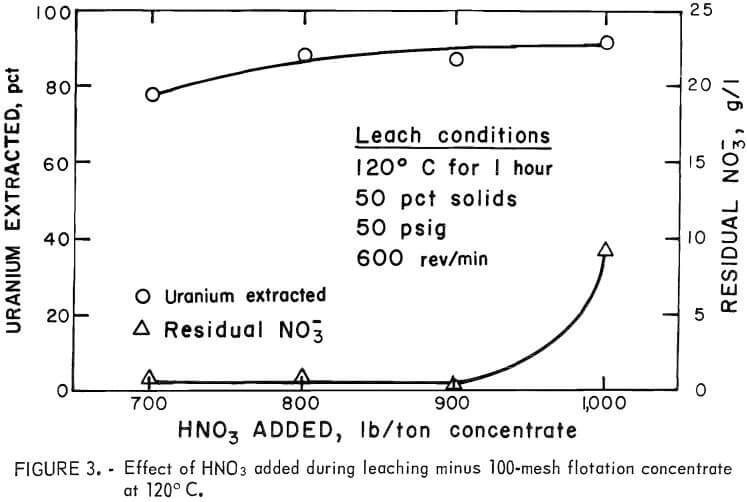
Because the HNO3 leaching reaction decomposes pyrite and degrades the carbonaceous matter, particle size may have some effect on the leaching properties of the ore. Consequently, samples of the minus 35-mesh concentrate were ground to 100 pct minus 100-mesh and leached with nitric acid. It is clear after comparing figures 2 and 3 that finer grinding did not improve the uranium extraction, and that more acid was required to achieve similar extraction levels. The latter effect was probably due to a more complete reaction with the pyrite and dissolution of the small amount of iron unavoidably added to the concentrate during size reduction.
Effect of Time and Temperature
The experimental apparatus and procedure were modified slightly in these experiments to permit continuous pumping of acid into the reactor. This was important for temperature control because it prevented the 50 to 10° C temperature excursions common in previous experiments after each aliquot of acid was injected into the reactor. Four hundred grams of ore and 400 ml of water were heated to operating temperature in the reactor. Then all of the nitric acid was pumped into the reactor during the next hour, followed by 3 hours at temperature for completion of the reaction. In these experiments, the temperature was maintained within ±2° C of the desired values. Samples for uranium analysis were taken after 0.5, 1.0, 2.0, 3.0, and 4.0 hours; each residue was
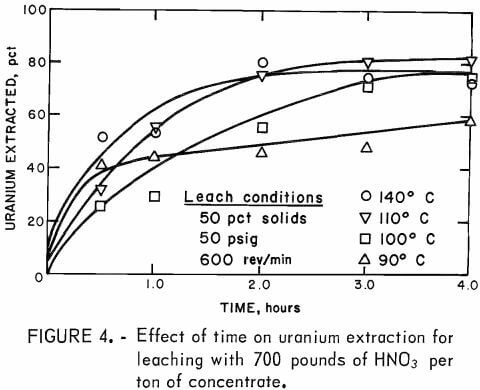 washed thoroughly to remove all soluble uranium and then analyzed. Figure 4 shows the results of these experiments. Temperatures of 110° to 140° C were required for the highest uranium extractions. The lower extractions obtained at 90° and 100° C were probably due to decreased attack on the pyrite mineralization by the leaching agent. From a practical operating point of view, leaching at 100° to 110° C with 700 pounds of HNO3 per ton of concentrate for up to 4 hours would be most desirable.
washed thoroughly to remove all soluble uranium and then analyzed. Figure 4 shows the results of these experiments. Temperatures of 110° to 140° C were required for the highest uranium extractions. The lower extractions obtained at 90° and 100° C were probably due to decreased attack on the pyrite mineralization by the leaching agent. From a practical operating point of view, leaching at 100° to 110° C with 700 pounds of HNO3 per ton of concentrate for up to 4 hours would be most desirable.
Based on data obtained from these experiments , failure of the HNO3 leach system to completely solubilize the uranium is unexplainable; however, it is likely that from 15 to 20 pct of the uranium was locked as highly refractory silicate and uranium-vanadium minerals.
Offgas Analysis
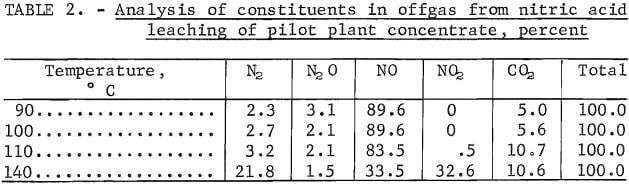
Gas analyses were conducted on the offgases from the 90°, 100°, 110°, and 140° C leach experiments run with 700 pounds of HNO3 per ton of ore. The analysis procedure was to collect the gas in a 100-cm³ syringe and inject it into a gas chromatograph. Gas analysis of these experiments is shown in table 2. These results indicate that the reactions proceeded approximately as postulated only at the lower temperatures. The 140° C reaction behaved contrary to predictions. The presence of NO2 indicates that the temperature of 140° C was hot enough to “boil” off some of this constituent before it could react with the ore. The presence of nitrogen and N2O in the offgases from all of the experiments indicates that some of the nitrate was reduced beyond the NO stage. These degradation products must be considered as losses of nitrate from the system because they cannot be reconstituted to nitric acid.
Nitrate Losses
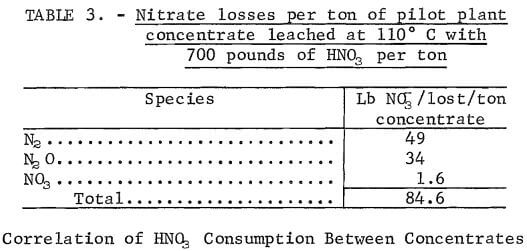
Table 3 shows the nitrate losses to be expected due to undesirable side reactions that occur when leaching the pilot plant concentrate at 110° C with 700 pounds of HNO3 per ton of ore. These results indicate that reduction of nitrate to nitrogen and nitrous oxide is highly detrimental. Further research is warranted to minimize this loss. However, solution nitrate losses would be negligible.
Analysis of leached concentrate showed that 3 pct of the organic carbon reacted with the nitric acid. Because the flotation concentrate produced in the laboratory contained 16.6 pct carbon versus 9.7 pct in the pilot plant concentrate, the additional 6.9 pct carbon would increase the amount of carbon to be oxidized by 4.1 lb/ton of ore, or
0.03 x 6.9 x 20 lb C/pct/ton = 4.1 lb C/ton.
As shown in equation 2, 2 moles of HNO3 react with 1 mole of carbon; thus 43 pounds of HNO3 would be required to react with the carbonaceous matter contained in 1 ton of the laboratory-produced concentrate, or
4.1 lb C/12 lb-mole C x 2 x 63 lb-mole HNO3 = 43 lb HNO3.
Similarly, 61 pounds of HNO3 per ton would be required for the pilot plant concentrate, or
0.03 x 9.7 pct x 20 lb C/pct/ton x 2 x 63 lb-mole HNO3/lb lb-mole C = 61 lb HNO3/ton
Assuming that the remainder of the nitric acid reacts with the pyrite, 639 pounds of HNO3 per ton would react with 6.4 pet sulfide sulfur, or
700-61 = 639 lb HNO3/ton
This is 100 pounds of HNO3 per pct of S2- per ton on a percent-sulfide basis. The laboratory concentrate contained 6.1 pct S2- so that 610 pounds of HNO3 per ton would be required by the sulfides , or
6.1 pct S2- x 100 lb HNO3 /pct S2-/ ton = 610 lb HNO3/ton
Thus, the carbonaceous matter in 1 ton of laboratory concentrate would react with 43 pounds of HNO3, and the sulfide sulfur content would react with 610 pounds of HNO3 to give a total nitric acid requirement of 653 pounds of HNO3 per ton of laboratory concentrate.
Correlation of nitrate losses from one concentrate to the other is difficult because the mechanism for nitrate reduction during the leach step is not known. Therefore, the nitrate loss value observed for the pilot plant concentrate was used in calculating HNO3 requirements for the laboratory concentrate. On this basis, the NO retained in the system would be sufficient to regenerate 568 pounds of HNO3 per ton of concentrate treated.
Solution Concentration and Purification
Resin ion exchange and solvent extraction methods were tested for concentration and purification of uranium from the nitric acid process leach solution. Although these methods have proved to be versatile and economical in commercial practice, their effectiveness in treating solutions from highly carbonaceous ores has often been impaired by the presence of partially oxidized carbonaceous constituents in the leach solution. To determine the probable extent of fouling that might occur during concentration and purification, resin and solvent extraction loading capacities were investigated on a cyclic basis with 0.15 g/l nitrate content leach solutions generated by nitric acid leaching of flotation concentrate. In effect, this was the worst case approach since the impurity content of this leach solution would in practice be diluted eightyfold when combined with the solution from conventional sulfuric acid leaching.
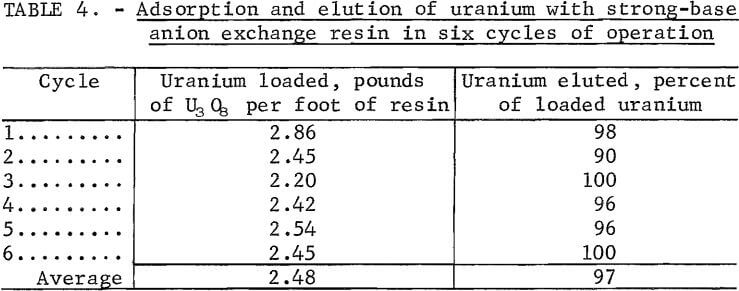
Strong base anionic exchange resins were evaluated for their uranium-loading capacities from the leach solution. Loading values as high as 2.86 pounds of U3O8 per cubic foot of resin were recorded, but a noticeable darkening of the resin was observed. It was concluded that the color change was due to unknown dissolved organic species from the leach solution occupying resin sites because adsorbed iron and nitrate were eluted with the uranium from the resin.
Chemical regeneration of the resin restored the original resin color as well as the uranium-loading capacity, but it was considered important to determine the effect of the dissolved organic species on the resin in continuous-loading and eluting operations without the benefit of regeneration. Table 4 shows the results of a six-cycle loading and stripping experiment. The feed solution was at a pH of 1.5 and contained 1.39 grams of uranium per liter.
The initial uranium loading was 2.86 pounds of U3O8 per cubic foot of resin with fresh resin. Loading dropped to 2.45 pounds of U3O8 per cubic foot of resin at the second cycle, or a decrease of 13 pct. Upon continuing the operation, however, the uranium loading was relatively constant. These results indicate that a certain degree of resin poisoning by the dissolved organic species did occur initially, but the condition appeared to reach a steady state. The average result for uranium loading was 2.48 pounds of U3O8 per cubic foot of resin. An average of 97 pct of the loaded uranium was stripped with nine bed volumes of eluting agent.
A tertiary amine solvent extraction system was chosen to extract uranium from the leach solution because of its high selectivity and loading capacity. Ferric iron in the uranium solution is effectively rejected by amines in contrast to its adsorption by the anionic resins.
In preliminary tests, the uranium extraction coefficient, Eu°, was determined as a function of the phase ratio, pH of the leach solution, contact time, and composition of the organic extractant. The best extraction results were obtained using an organic phase composed of 5 vol-pct triisooctylamine,
3 vol-pct decanol, and 92 vol-pct kerosine, a pregnant aqueous solution pH of 1.25, organic-to-aqueous ratio of 1:1, and contact time of 1.5 min. Under these conditions, the Eu° was 80, and 99 pct of the uranium was extracted. Approximately 97 pct of the loaded uranium was stripped in a single operation. Iron was not extracted with the uranium into the organic phase and remained in the raffinate. Nitrate ion was extracted into the organic phase but was completely stripped.
Durability of the amine as an extractant for uranium was determined in a semicontinuous extraction and stripping operation. In each operation cycle, the organic phase was loaded twice and then stripped twice. The raffinate from the second stage of extraction was cycled to the next stage, where the remaining uranium was extracted by the recycled barren organic phase. The eluant from the second stage of stripping was diverted to the next stage to meet with freshly loaded organic phase. The leach solution at pH 1.0 contained 17 grams of uranium per liter. No change in loading capacity or color of the organic phase was observed. This 10-cycle test showed that uranium loading of the amine was unaffected by the dissolved organic species in contrast to the lowered loading level observed for the resin.
Proposed Operating Conditions
A flow diagram incorporating nitric acid and conventional leaching is shown in figure 5. Based on a 2,000-tons/day ore feed and a 1:80 concentrate-to-feed ratio, 25 tons/day of concentrate would be produced requiring 16 tons/ day of H2SO4, 0.8 ton/day of xanthate collector, and 0.05 ton/day of frother. Sulfuric acid leaching of the flotation tailings would require an additional 34 tons/day of H2SO4. Nitric acid leaching would require 1.1 tons/day of fresh HNO3 and 7.1 tons/day of HNO3 regeneration capacity. After leaching at
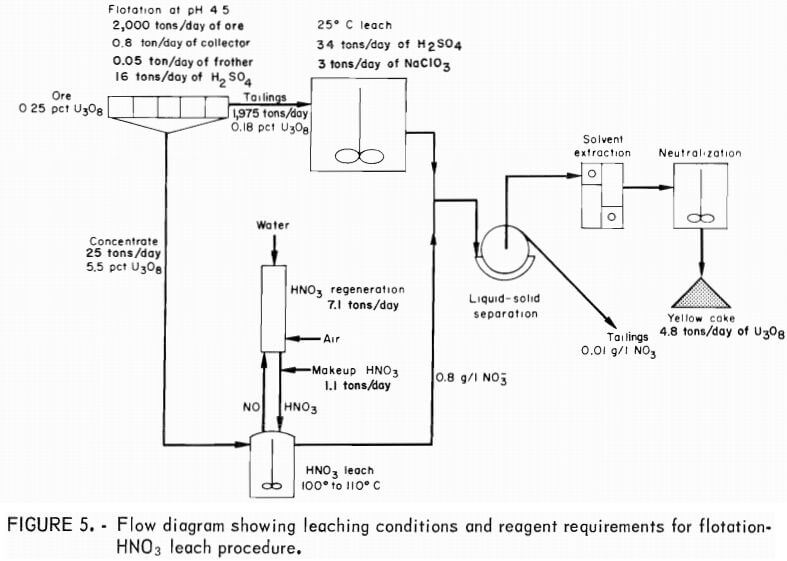
100° to 110° C, the two leached slurries would be combined into a common slurry for conventional ion exchange or solvent-extraction processing.
Using the flotation-nitric acid leach technique for this ore would increase the uranium extraction from 85 pct for conventional H2SO4 leaching to 96 pct, or
85 pct + 0.95 x 0.80 x (100-85 pct) = 85 pct + 11 pct = 96 pet
In terms of extra pounds of uranium recovered per day, based on an ore feed concentration of 5.0 pounds of U3O8 per ton, an additional 1,100 pounds of U3O8 would be recovered each day from this ore, or
0. 11 x 2,000 ton/day x 5.0 lb U3O8/ton = 1,100 lb U3O8/day.
Energy and Environmental Considerations
This procedure could require less energy when compared with conventional acid leaching because some mills have installed extra steam capacity for heating to as high as 60° C to increase throughput by faster leach-rate kinetics. Flotation reduces the amount of pulp that must be heated in the nitric acid part of the leach by a factor of 80. It would require approximately one-twentieth as much thermal energy to heat the concentrate to 110° C as to heat the entire ore to 60° C. Furthermore, laboratory results show that once the leaching temperature has been achieved, the leaching reaction can conceivably produce sufficient energy to maintain this temperature.
Offgases from the reactor-regeneration system are environmentally acceptable with the exception of N2 O, which would have to be catalytically converted to N2. The effluent from nitric acid leaching would contain approximately 0.8 g/l NO3. When combined with the other process stream, the plant effluent would contain 0.01 gram of NO3 per liter or 10 milligrams of NO3 per liter, which is well below the drinking water quality standard of 44 milligrams of nitrate per liter. Unfortunately, as is almost universally the case , dissolved iron, vanadium, radium, and molybdenum would exceed effluent guidelines; therefore, the solutions would have to be impounded.
Conclusions
A procedure was devised for increasing the overall recovery of uranium from 85 to 96 pct for an ore containing refractory carbonaceous and sulfidic mineralization. This technique employed froth flotation and sulfuric acid and nitric acid leaching. The following conclusions were drawn based on laboratory results:
- Up to 95 pct of the refractory uranium can be concentrated by flotation of minus 35-mesh ore with 0.80 lb/ton potassium ethyl xanthate collector and 0.05 lb/ton frother.
- Nitric acid leaching at 110° C for 4 hours extracts 80 pct of the uranium from the flotation concentrate.
- Nitric acid requirements were calculated to be 653 lb/ton of concentrate.
- A total of 568 pounds of HNO3 per ton of concentrate can be regenerated by reaction of offgases with air and water.
- The leaching reaction is temperature insensitive in the operating region.
- Low operating temperatures favor nitrate conservation.
The Bureau of Mines investigated a flotation-nitric acid leach procedure as part of the goal to maximize minerals and metals recovered from primary and secondary domestic resources. Studies were conducted on an ore that contained carbon-bearing and sulfide mineralization that rendered a portion of the ore refractory (resistant) to conventional leaching technology. The procedure investigated for treating the ore consisted of the following: (1) separation by flotation of the carbonaceous and sulfidic components from the ore, (2) leaching the flotation concentrate with nitric acid at 100° to 110° C, (3) leaching the flotation tailings with sulfuric acid, and (4) processing the combined leached slurries in a conventional manner to recover yellow cake. In step 2, HNO3 is converted to gaseous products from which it is regenerated by reacting these products with air and water for further leaching. An overall uranium extraction of 96 pct was achieved by this procedure.
