Develop methods of enhancing and measuring the high-temperature softening and melting properties of iron oxide pellets reduced under simulated blast furnace smelting conditions.
Add dolomite and limestone flux and a low-cost organic binder, such as starch, carboxyl methylcellose (CMC), or waste papermill sludge, to the iron oxide concentrate to produce hematite (ferric oxide) pellets with superior high- temperature metallurgical properties. Simultaneous increases in softening temperature, gas permeabilily, and enhanced metallization rates are sought to improve blast furnace productivity and energy efficiency.
In the past, bentonite, an inorganic binder, was the only additive (binder) used in the production of pellets made from iron ore concentrate. Research has shown that the reducibility of conventional (acid) pellets can be increased by the addition of flux or an organic binder. The increase in pellet reducibility results in less wustite in the center of the pellet at high temperatures, such as those obtained in the cohesive zone of the blast furnace.
Design Procedure
The Bureau has constructed a high-temperature, softening-melting testing apparatus for evaluating the behavior of pellets under simulated blast furnace conditions. This apparatus is very effective in evaluating the contraction and pressure drop of fired pellets made with different additives, such as flux, bentonite, CMC, starch, or waste papermill sludge. The fired pellets are placed between two coke layers in a specially designed furnace. A 0.5-kg/cm² load is placed on the top coke layer. A reducing gas mixture is injected into the bottom of the multi layer bed. Bed deformation and pressure drop across the bed are continuously measured as the temperature is increased. Monitoring continues until the molten iron and slag drain out of the furnace. In quenched tests, the furnace is shut down at a preselected temperature to determine the degree of pellet metallization and deformation.
How It Works
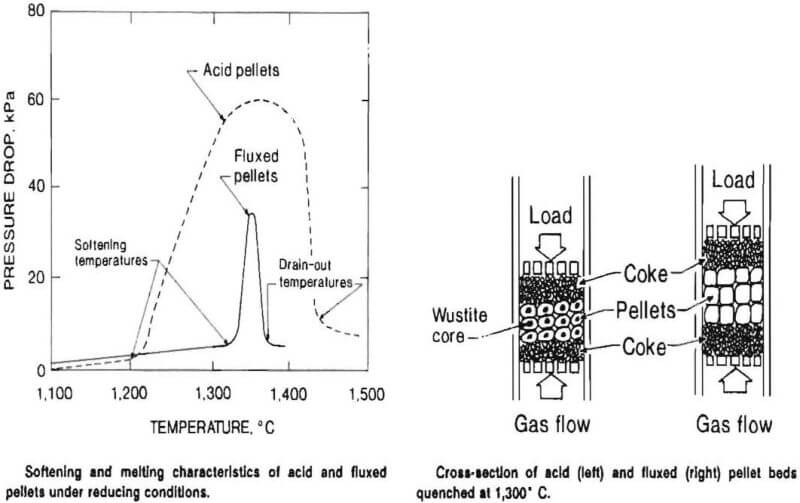
The pellet and coke layers are heated under load to reduce the hematite to metallic iron. When the pellets are partially reduced, they soften and deform and the gas pressure drop across the pellet bed increases. The definition of the softening temperature is the temperature at which the pressure starts to increase significantly. The drain-out temperature is defined as the temperature when the pressure drop reverts the baseline.
Investigative Results
Acid pellets softened at temperatures below 1,300° C and a large pressure drop was observed between about 1,300° and 1,400° C. Pellets made with flux (4 pct dolomite and 4 pct limestone) or with an organic binder (0.5 pct papermill sludge or 0.1 pct starch) had a higher softening temperature and a lower pressure drop than acid pellets. With fluxed pellets, a lower drain-out temperature was also observed.
In some tests, pellet beds were quenched at 1,300° C. These tests showed that more wustite and fayalite (ferrous silicate) was present in the center of the acid pellets as indicated by a dark core. The acid pellets had a dense outer shell of metallic iron. With fluxed pellets, the metallic iron did not coalesce to form a dense shell, thereby enhancing the reduction of the iron oxides. Also, the calcium and magnesium in the pellet tied up the silica and minimized fayalite formation.
For More Information
Details of the pellet binder research are available in Bureau Report of Investigations (RI) 9230, “Effectiveness of Organic Binders for Iron Ore Pelletization,” and RI 9257 “Utilization of Papermill Sludges as Binders for Iron Ore Concentrate.” Additional details concerning the fluxed and acid pellet research are available from the principal investigator:
