The principle oxide minerals of molybdenum are wulfenite (PbMoO4), ferrimolybdite (Fe2(MoO4)3·nH2O), powellite (CaMoO4), and ilsemannite (Mo3O4). Wulfenite is found in Arizona, New Mexico, Nevada, Utah, California, and Montana. It is also found in the states of Sonora and Chihuahua, Mexico. Prior to 1915 wulfenite ores were the primary source of molybdenum.
All leaching experiments were performed in a thermostated glass flask (resin kettle with baffles). Exactly 500 ml of Na2S solution of the desired concentration were prepared fresh for each experiment and were placed in the reaction flask. One gram of PbMoO4 was added to the solution when the operating temperature had stabilized.
Wulfenite dissolves in the presence of sulfide ion according to the following reaction
PbMoO4 + S= ↔ PbS + MoO4=
The rate data are expressed in terms of the fraction of molybdenum dissolved, which is based on the analysis of molybdenum in solution and the amount of molybdenum in each wulfenite sample.
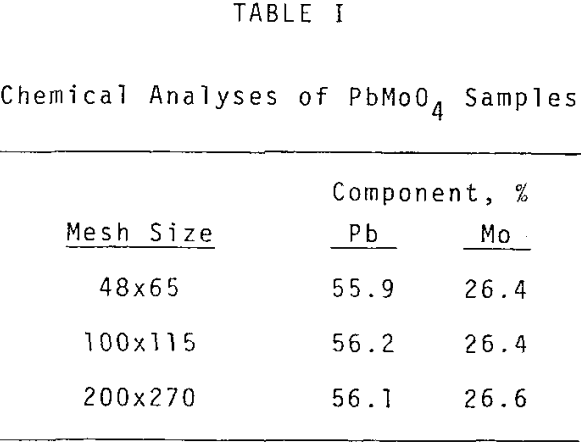
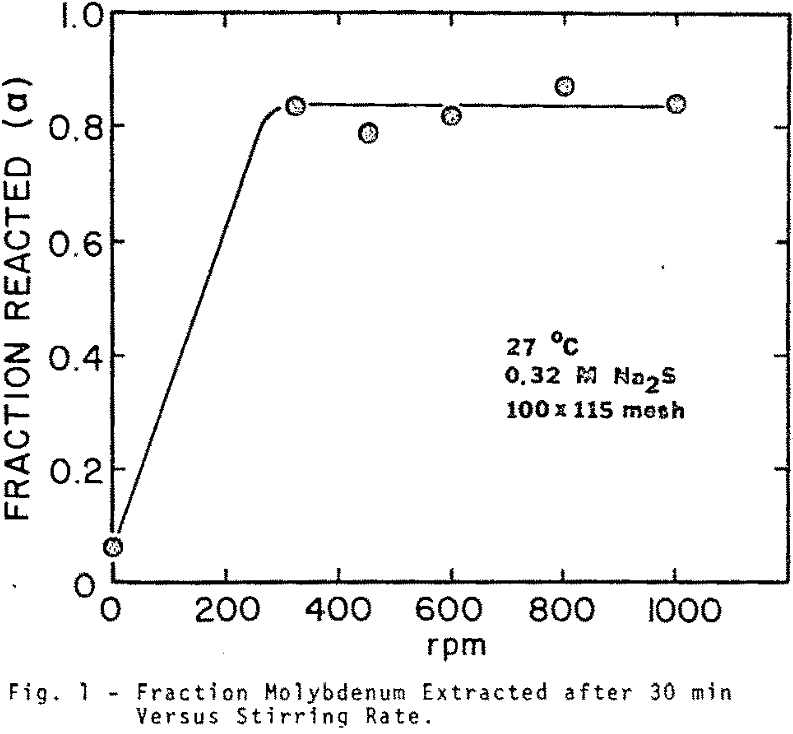
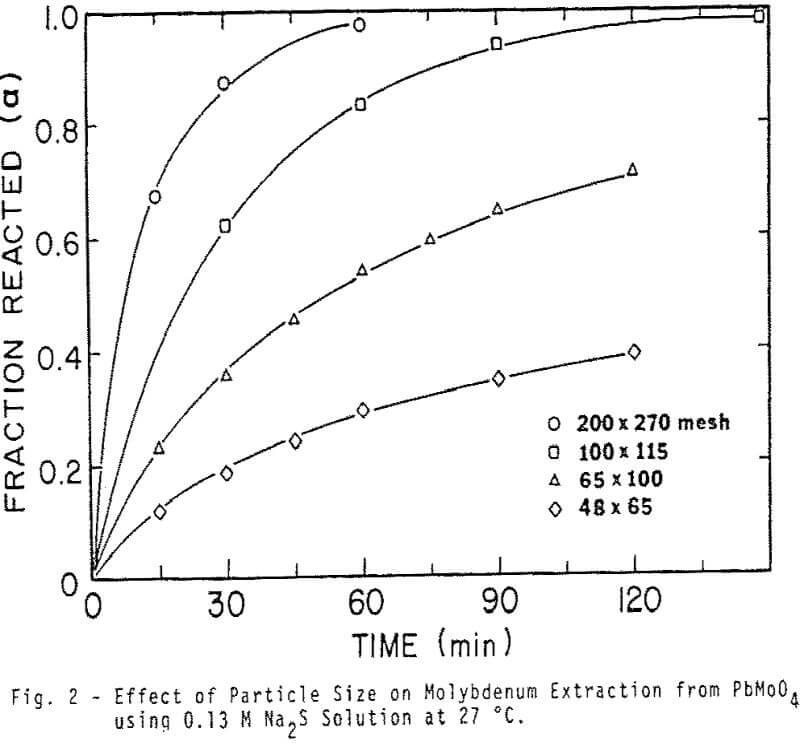
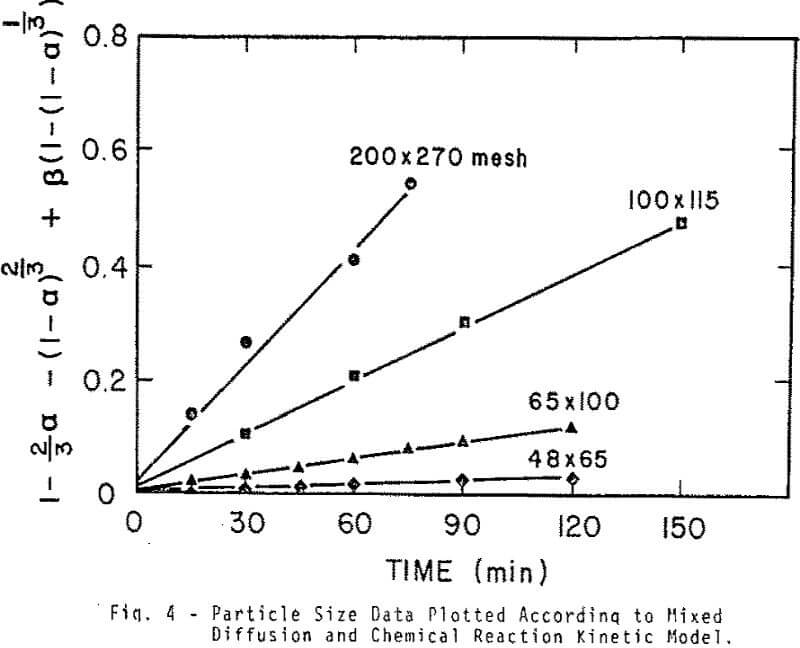
Sized PbMoO4 ranging from 35×48 to 200×270 mesh was used for this aspect of the investigation. The rate of extraction is noticeably affected by particle size over the range investigated. The shape of the extraction curves is typical of a shrinking core reaction where reactant surface area is decreasing and diffusional path lengths are increasing.
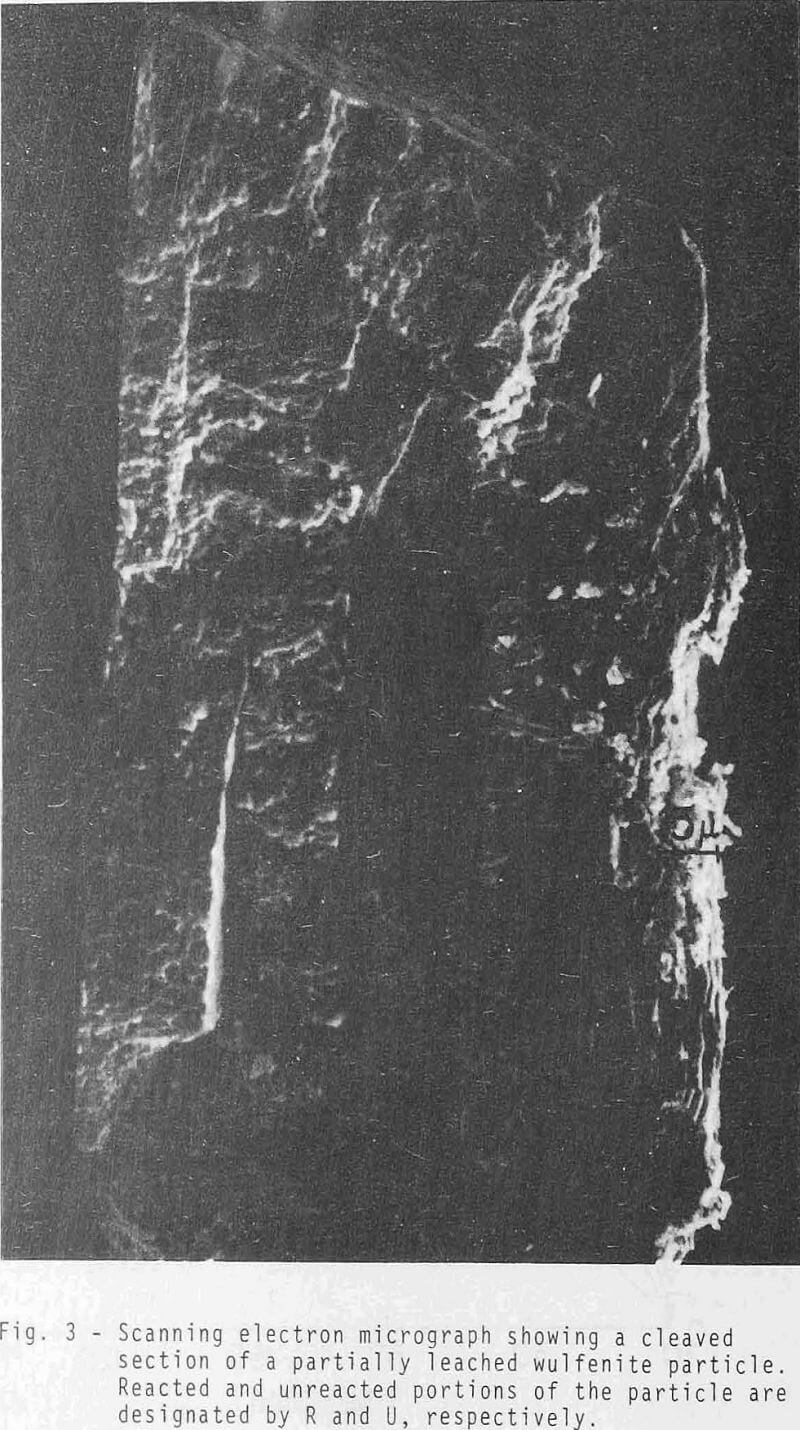
The kinetic results were initially analyzed in terms of a reaction model involving just diffusion of reactant inward through the PbS product layer. Experimental data correlated well with the relationship developed by Valensi for diffusion through a spherical product shell.
Effective diffusivities and chemical reaction rate constants were calculated. The sphericity and boundary roughness term is assumed to be unity. The molar volume of PbMoO4 is 53.9 cm³/mole and the concentration term is assumed constant and is taken to be equal to the sulfide ion activity. Bi-sulfide and sulfide ion concentrations were calculated from the following ionization equilibria of H2S.
The extraction of molybdenum from 48×65 mesh wulfenite at 50°C was determined for different concentrations of sodium sulfide. Extraction curves indicate that the leaching is extremely sensitive to sodium sulfide concentration in the range between 0.032 and 0.32 M Na2S.
The reaction model involves the following sequence of steps:
- Boundary layer diffusion to the particle Surface,
- Diffusion of sulfide ion through the PbS product layer
- Chemical reaction between the sulfide ion and the unreacted PbMoO4 core
- Diffusion of molybdate ion outward through the PbS product layer.
- Diffusion of molybdate ion through the boundary layer into the bulk solution.
It is reasonable to neglect the contribution of boundary film diffusion because of strong agitation. Furthermore, Steps 4 & 5 can be disregarded because the reaction is irreversible. It is proposed that the rate is controlled by a mechanism involving both the diffusion of sulfide ion inward through the PbS product layer, followed by chemical reaction at the PbMoO4 boundary.