- To participate in the 911Metallurgist Forums, be sure to JOIN & LOGIN
- Use Add New Topic to ask a New Question/Discussion about Mineral Processing or Laboratory Work.
- OR Select a Topic that Interests you.
- Use Add Reply = to Reply/Participate in a Topic/Discussion (most frequent).
Using Add Reply allows you to Attach Images or PDF files and provide a more complete input.
- Use Add Comment = to comment on someone else’s Reply in an already active Topic/Discussion.
-
https://www.911metallurgist.com/wp-content/plugins/wp-symposium-pro/forums/../css/images/wait.gif
Substitute of sodium hydrosulfite in bleaching of talc ore. (1 reply)
Please join and login to participate and leave a comment.

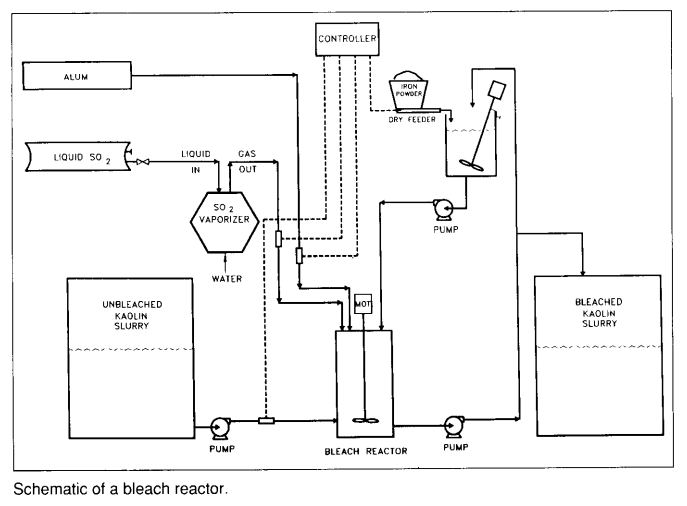
Hello,
Sodium hydrosulfite (or sodium dithionite) is an excellent reducing agent. It acts as a hematite reductant in bleaching process of talc; the reaction is very fast at room temperature. The problem is that the talc becomes contaminated with sulfur compounds. Can anyone suggest another bleaching agent?
Sorry, I don't speak english.
Thank you for understanding!