Table of Contents
The dry methods of assaying mercury-ores and other combinations of mercury all rest upon the volatility of this metal as a beginning.
After the separation of the mercury in the form of vapor from the matrix upon which the assay is performed, the mercurial vapors are either condensed upon cold surfaces or received upon a gold plate into which the mercury is absorbed.
In the first case the product is recovered and weighed as metallic mercury in an isolated state.
The distillation-processes are practiced in either earthen or iron retorts, sometimes made of gas-pipes or in other provisional ways, or in glass tubes and upon quantities varying from a kilogramme or two down to a few grammes.
In any case the results of an ordinary distillation-assay are sure to be uncertain and unreliable, through loss of mercury, for close work. There is a great deal of difficulty in sealing all the joints of the apparatus, so as to prevent the escape of mercurial vapor. The metallic mercury after condensation is, at least in part, divided into minute globules, which are very elusive and frequently include particles of dust or other foreign bodies.
As a result the distillation-processes ought to be, and probably are, relegated to the class of “ rough tests,” in which capacity they are very useful. The sight of the metallic drops has often loosened the purse-strings of the doubting investor.
The production of mercury on a large commercial scale is well known to depend upon the utilization of ores of very low grade; and where this is practiced, it long ago became necessary to discard the distillation-methods of assay in favor of others that would give more exact results and admit of closer comparisons.
Such are the assays in which the mercury is determined in combination with gold; there being no simple wet assays that give practical results comparable with this class of dry assays.
The assays with gold are practiced upon quantities of ore ranging from 10 to 2 grammes; the richer the ore the less of it should be used. Never should there be so much mercury in the charge that the distillate hangs in drops upon the recipient.
The sample is mixed with iron, lime, litharge or other desulphurizing flux, and is placed in a porcelain crucible, which is then closed with a close-fitting concave cover, made of fine gold. The concavity of the cover is filled with distilled water, and the lower part of the crucible is then carefully heated. The mercury is volatilized, and deposits upon the gold cover as a white stain, like frosted silver.
When the operation is supposed to be ended, the cover is removed, carefully washed, dried in a desiccator, and then weighed. At the beginning of the operation the cover has also been weighed, so that the increase of weight in the second weighing is due to the presence of mercury. Thus the percentage of this metal in the original charge of ore is easily ascertained.
This is, theoretically and practically, almost a perfect method. Repeated trials have shown agreements in duplicate assays to within 0.04 per cent, on poor ores, containing less than 0.5 per cent, of mercury, and within less than 0.5 per cent, on ores containing as high as 30 per cent, of mercury.
Only the very best analysts would be likely to get closer results with the wet methods at greater expenditure of time and money and no corresponding practical gain.
However, the gold-cover method has its drawbacks. The cover is expensive; scarcely less than an ounce of gold can be used if the cover is to stand many assays, for fine gold is quite soft, and the cover must be stiff enough, and have body enough, to stand handling without bending.
After the assay is finished, the mercury must be driven out of the cover by heat; and if the heating is not most carefully done, some of the gold will volatilize with the mercury. Notwithstanding all precautions, the gold cover, after a few determinations, begins to get spongy and afterwards to flake off. There is a considerable loss of weight; the assays are uncertain and discrepant in their results; and at last the cover must be remelted with new gold added, and then rerolled and rewrought.
Finally, the visible presence of gold is a temptation to irresponsible helpers and chance visitors. When goldsmiths are employed to form the covers, there is no security of receiving back all the gold with which they may have been furnished; or, if they themselves furnish the gold, their certificates, verbal and written, often cover more copper than the analyst knows of or desires.
There was, some time ago, a perfect mania in this republic for quicksilver-discoveries. My assay-office was often full of samples, generally barren, but all firmly believed to contain unbounded quantities of the liquid metal. Under these circumstances I early turned from leaky gas-pipe retorts and brittle glass tubes, and found a certain measure of relief in the gold-cover method of assay.
The drawbacks of the gold-cover are, however, strongly accentuated when one employs or comes into contact with persons whose ideas about portable property do not strongly distinguish between meum et tuum. Hence I sought for a method not so committed to the use of articles of high value, and thought first of using silver in the place of gold.
I have never seen any statement of the comparative affinity
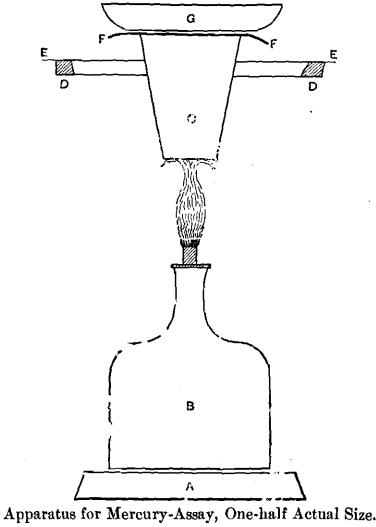
A Base of Retort Stand. B Spirit-Lamp. C Retort or Annealing-Cup. DD Retort-Stand Ring, which serves as support to the Apparatus. E E Tin Shield. F F Silver Foil for receiving the mercury. G Cooling-Cup.
of gold and silver for mercury, but possibly there is little difference between the metals in this respect, and perhaps silver has the stronger attraction of the two.
I desired also to get rid of the necessity of driving out the mercury by heat from the apparatus after every assay, and, lastly, I wanted to make a durable and cheap apparatus. The result of my experiments, as tested by considerable practice, is shown in the accompanying figure.
Recipient
The substance which I use to receive the vaporized mercury is, as I have before indicated, pure silver, and I use it in the form of foil, such as is commonly sold by dealers in assayers’ supplies.
By actual measurement with a micrometer, the foil which I have been using is 0.02 millimeter in thickness. It costs in the United States about 5 cents gold per gramme.
At each assay I use a piece about 5 centimeters square, weighing about 0.6 gramme, so that the actual cost for each assay is about 3 cents, if the foil is not used over again. But it can be used, with very ordinary care, several (at least three) times, so that the cost becomes less than one cent per assay.
Refrigerator
For the purpose of cooling the silver-foil I make use of a silver dish of a wide pattern like an evaporating-dish. This form was adopted precisely for the reason that it facilitates the cooling for which it is designed. I chose silver because it has the highest heat-conducting power of all the metals, but copper approaches it so very nearly in that respect that a copper dish would practically be just as good, while it would cost less. The silver used in this dish is coin-alloy about 900 fine.
The dimensions are: 5.5 centimeters diameter at bottom, 6.5 centimeters at the top, and height somewhat over 1 centimeter.
It holds, comfortably filled, a little more than 20 cubic centimeters of water, and weighs, empty, 29 grammes.
It is highly polished, and is kept so, especially on the bottom, so as to betray any particle of mercury that might soak through, during the determination, from the foil which is under it. However, this has not happened in a large number of assays, and is not likely to happen if proper care be taken in making the charge of ore. If it did happen, there would be no great harm done, as the mercury could easily be driven out of the dish by heat. This would involve, of course, the loss of that particular determination, which would have to be repeated with a smaller charge of ore.
The Retort
As a retort, if I may call it so, I use a Battersea annealing-cup, size C. This is a crucible of unglazed white clay, in the form of a truncated cone, 2 centimeters outside diameter at the bottom and 3.5 centimeters diameter at the mouth; the height is 4.5 centimeters. The mouth may be ground to an even surface in any convenient way, but generally this is not necessary.
Heat is applied to the bottom of the retort during the operation. To prevent the direct heating of the upper part of the crucible and of the silver-foil and cooling-cup, I make use of a circular tin shield, 13 centimeters in diameter, which has a hole in the center a little less than 3.5 centimeters in diameter. The annealing-cup will pass almost through this hole, and will remain firmly fixed therein, with about 1 centimeter of its upper part protruding.
The tin shield serves also to suspend the crucible and the rest of the apparatus from the ring of a retort-stand.
The annealing-cup used as a retort is of indefinite duration. The tin shield costs practically nothing.
Source of Heat
For heating the apparatus I use a small glass lamp, which holds about 60 cubic centimeters of alcohol when comfortably filled. The brass wick-tube is 6 millimeters internal diameter, and when in action the wick is regulated to produce a flame from 4 to 5 centimeters high.
Flux and Charge
For a flux I use iron-filings, the finer the better. Those I have in hand would go through a 60-mesh sieve. A long time ago I secured several hundred grammes of these filings, and prepared them for use as a flux by first lixiviating them with strong alcohol, to remove most of the grease, and then igniting the filings by heating them to redness for some time in a muffle. The filings are preserved for use in a glass bottle with a rubber stopper. The cost of this flux is practically nothing.
Probably litharge would do as well for a flux as the iron- filings ; but I have found the latter quite satisfactory, and have never used any other. The litharge might melt and spoil the cup, which the iron will not do.
The charge for a mercury determination is from one-half to one gramme of ore. This is intimately mixed with 5 grammes of the prepared iron-filings. The mixture is accomplished in the annealing-cup itself by means of a spatula. About one gramme of iron-filings is placed on the charge as a cover.
Operation
After the crucible or retort has been charged as above described, it is hung by its tin shield from the ring of a retort-stand.
A piece of silver-foil is then cut large enough to cover the mouth of the crucible and leave a good margin of, say, one-half centimeter all round. The foil is carefully smoothed, and then ignited in the flame of the alcohol-lamp. Care must be exercised, for the foil is so thin that it will fuse at once if allowed to overheat.
After cooling sufficiently, under cover, the foil is weighed on an analytical balance, and is placed upon the mouth of the crucible. It is then gently pressed down with the finger until it moulds itself to the shape of the mouth of the crucible.
The cooling-cup is then placed upon the crucible on top of the silver-foil, and is filled with water.
The alcohol-lamp is placed under the crucible, and is arranged to give a flame about 4 centimeters high, which shall just barely spread out at its point over the central part of the bottom of the crucible.
The heating should continue in this way for from 10 to 15 minutes. Ten minutes is too short for most ores, and anything over 15 minutes is apt to lead to loss of mercury. Over 20 minutes is, in most cases, fatal.
The water in the cooling-cup may be renewed once or twice during the heating; possibly it would be well to use ice-water for this purpose. Water at ordinary temperatures, however, has always given me good results.
When the heating is finished, the crucible and contents are allowed to cool at least five minutes. When the silver-foil is removed, a distinct mercurial stain will be seen upon its lower surface, if there was the slightest trace of mercury in the ore.
The amount and depth of this stain is a rough indication of the amount of mercury in the ore. The foil is conveyed (under cover, to avoid dust) to the balance.
The increase in the weight of the foil shows the amount of mercury absorbed; and a simple calculation gives the amount of mercury contained in the original charge of ore.
In order to check the first determination, and to make sure that all the mercury has been collected, I repeat the heating on the same charge for about ten minutes more, and then weigh again. If the weight is constant, or there is a slight decrease, the amount of mercury obtained by the first weighing may be considered correct. If more mercury has been absorbed on the second weighing I repeat the determination with a new charge and heat for a longer time, say from five to ten minutes longer, than at the first heating.
Accuracy
I use a very good Kohlbusch analytical balance which weighs down to 0.05 milligramme; but I generally only weigh to 0.1 milligramme. With the above apparatus a content of 0.01 per cent, of mercury is clearly appreciable. The stain produced by that amount of mercury is plainly visible on the foil.
Owing to the extreme flexibility of the foil, a number or a date can be easily marked with a blunt point of any kind upon the corner of any one of the pieces of foil, and this can be preserved for future identification.
Some of these foils have been preserved in my laboratory for several years, and still show the mercurial stain without apparent alteration, and with no perceptible loss of weight.
On carefully igniting the foil over an alcohol flame the mercurial stain disappears, and the foil can be used for other determinations, probably for an indefinite number of times, if desired.
This test for mercury, viewed as simply qualitative, is more positive and more easily applied than most of the qualitative tests given in the books with which I am familiar.
Any prospector provided with a few ounces of fine iron filings previously ignited on a piece of iron over a stove, a small crucible or annealing-cup, an ounce of silver-foil, a copper cooling-dish and a spirit-lamp, can test his ores for mercury in a very easy way. When the spirit-lamp is not at hand, the necessary heat can be had from a lump of ignited charcoal.
With the addition of a fairly good pocket-balance and weights for weighing the charges, quantitative determinations can be made, the foils being reserved for future accurate weighing. In the laboratory, several copper cooling-cups can be kept, and a half dozen or more mercury-determinations can be made at one time, using as many pieces of foil. Not many assayers are rich enough to keep several gold covers on hand; but with the silver-foil there is practically no extra expense incurred by increase of facilities.
Failures
These may arise from too high a heat or too long heating. Generally, however, they arise from the foil being badly adjusted to the mouth of the annealing-cup. If this adjustment be carefully made, there should be no perceptible escape of mercurial vapor.
Important Mercury Assay Procedure Details
I claim as original the use of silver for receiving the mercury. I am aware that this has been suggested before, but do not know that it has ever been carried out in practice.
The use of silver in the shape of thin foil, and the use of a separate vessel to cool the receiving surface by contact. By the combination of the thin foil and the cooling-cup the cost of the assay is materially reduced, and the foil can be kept for reference, if desired. All of this I believe to be quite new.
The surface of the pure silver-foil, if carefully ignited before exposure to the mercurial vapor, is extremely sensitive, and readily absorbs the slightest trace of mercury. The stain made by one one-hundredth of one per cent, upon the foil is as visible as the deposit of moisture left by breathing upon a window-pane.
Those analysts and assayers who may have been using gold covers will no doubt discard them after a trial of the present method, and I would respectfully suggest that all such covers should be sent to me, and I will undertake to see that their value is properly expended in the cause of science !
