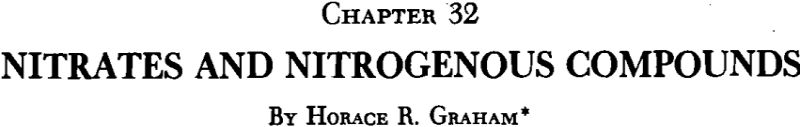Table of Contents
Chemical nitrogen and the “nitrates” of commercial significance are derived mainly from three basic sources: (1) the natural deposits in the form of nitrate-bearing earth and clay, which, being largely water-soluble, can exist only in the most arid portions of the earth; (2) coal, which yields not only nitrogen but also the additional by-product hydrogen required for the synthesis of ammonia and its compounds; (3) the atmospheric air, which contains 75.5 pct by weight of pure nitrogen gas.
Natural Nitrate Deposits
Occurrence
Although natural nitrate deposits consisting principally of the salts of sodium and potassium have been reported in Egypt, South Africa, Mexico, Argentina, Colombia, Peru and the USA, the only known natural nitrate resources of commercial importance are the vast deposits found in the Atacama, Tarapaca and Antofagasta regions of northern Chile, lying in a narrow strip 10 to 50 miles wide and roughly 450 miles long, between latitudes 19° and 26°S. In addition to yielding nitrates of sodium and potassium, these deposits are the source of the major portion of the world’s supply of iodine.
The nitrate-bearing ores known in Chile as caliche are variable in composition and may contain from 5 to 30 pct each of nitrate, chloride and sulphate. While the deposits are present chiefly in the form of the sodium salt, potassium, magnesium and calcium also occur in percentages from 0.1 to 5; usually as double salts with sulphate, which are but slightly soluble in nitrate leach solutions. These double salts are bloedite (Na2SO4.MgSO4.4H2O), glauberite (Na2SO4.CaSO4), polyhalite (K2SO4.MgSO4.2CaSO4.2H2O). Some deposits contain up to 30 pct of their nitrate content in the form of a double salt called Darapskite (NaNO3.Na2SO4.H2O), which is insoluble in cold leach solutions but is completely decomposed and soluble at elevated temperatures; i.e., 55°C and higher. Minor salts such as potassium perchlorate, sodium iodate and unknown borate materials, exist in percentages from 0.02 to 1. The water-insoluble salts may vary from 20 to 80 pct in the nitrate minerals.
Prospecting and Mining Practice
Inasmuch as the caliche is usually found close to the surface, and in relatively small thicknesses, prospecting and exploration are rather simple. The present practice is to sink pits one meter in diameter in promising localities and cut a good-sized sample channel vertically in each, sectionalizing the samples according to the variations in the ground, so as to get as good a classification of overburden, ore and under-lying material as possible. Pits are first sunk at wide intervals—perhaps 500 meters or even 1 kilometer—and then at closer intervals when promising areas are found..
Extraction of Nitrate and Preparation for Market
Sodium nitrate is now extracted from caliche in two types of plants. Until 1926, the only extraction plants in operation, of which about 150 were in existence, were known as the Shanks plants or Oficinas. In Shanks Oficinas, the caliche is brought from the mine, known as the pampa, in small cars up to 10 tons capacity, and crushed in jaw crushers to about 2½-in. maximum size and finally is delivered to boiling tanks
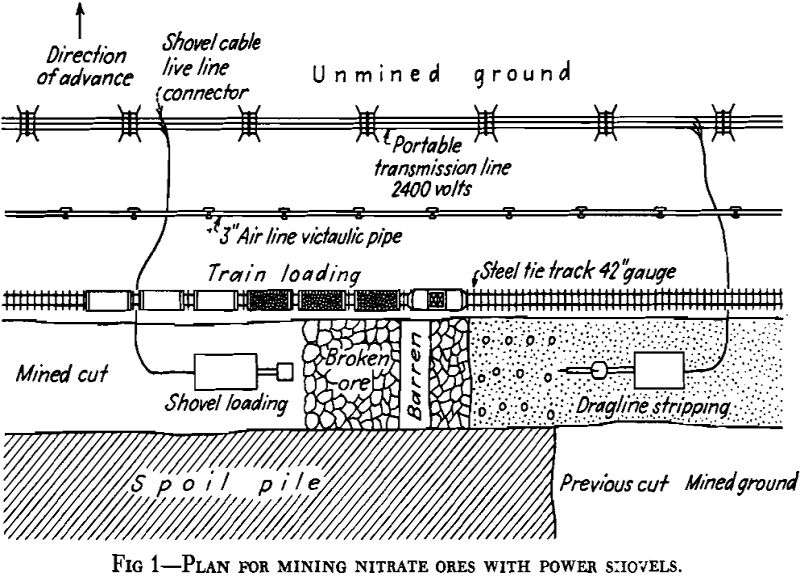
of 50 to 90 tons capacity. In these tanks, the caliche is leached at the boiling temperature with a mother liquor containing about 450 grams per liter of nitrate.
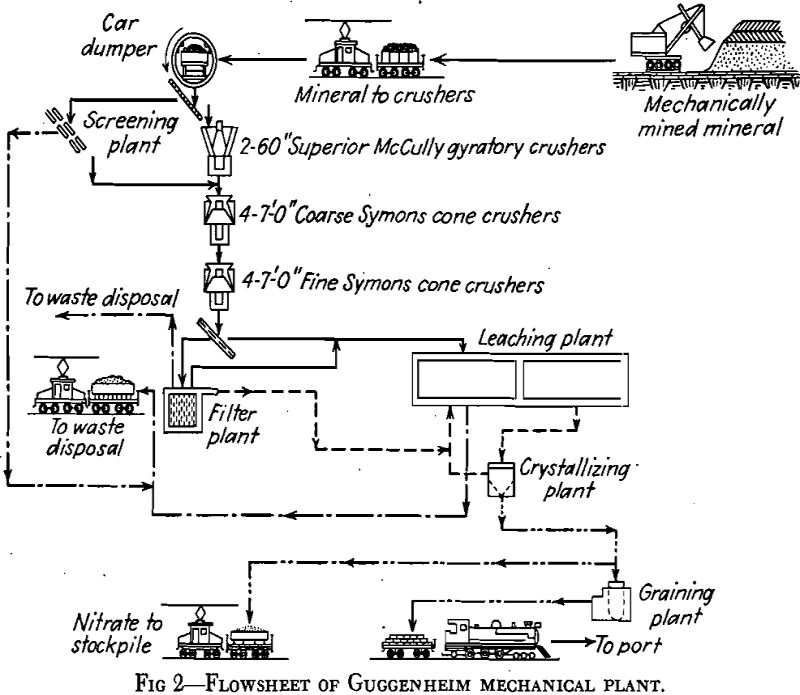
In the filter plant—of the Moore leaf type—the fines are mixed, 2 tons of fines to 1 cu meter of hot mother liquor to make a pulp of 60°C and 1.8 density. The pulp is then ready for filtering. When cakes 1 to 1½ in. thick have been formed, the filter baskets, each having a capacity of 25 tons of cake, are transferred to a brine-wash tank for a displacement wash. Water cannot be used because of serious pitting of the cakes and, therefore, poor washing. The final washed filter cakes contain 3 to 4 pct nitrate (mostly due to the nitrate content of the brine used for washing) and are discharged into water and pumped to waste.
Crystallized nitrate sludge from the crystallizers is delivered to a battery of 24 centrifuges for dewatering. The product is pure white, about 48-mesh in crystal size; it is of 95 to 96 pct purity and contains 3.0 to 3.5 pct moisture. While mother liquor from the crystallizing plant emerges at 35 °C, leaching temperatures throughout the plant are at 40°C. The extra heat required to heat the mother liquor as well as the caliche, and to provide for heat losses from the large open vats, is obtained as waste heat from the diesel power plant.
Extraction of Iodine
Iodine as an iodate dissolves in the nitrate leach solutions and builds up to concentrations of from 5 to 10 grams per liter. By the addition of sodium bisulphite (a local product made from sulphur dioxide and soda ash) the iodate is reduced to iodide. Then exactly one fifth more of the original solution containing iodate is added and solid iodine is formed, which settles to the bottom of the reaction tanks:
2NaIO3 + 6NaHSO3 = 2NaI + 3Na2SO4 + 3H2SO4
5NaI + NaIO3 + 3H2SO4 = 3Na2SO4 + 3H2O + 3I2
The spent liquor is returned to the nitrate plant and the iodine sludge is drawn off, filtered, pressed into “cheese cake” and retorted, delivering a crude product consisting of blue-black, lustrous crystals of about 99.4 pct purity. This product usually is sublimed by purchasers for delivery to consumers. The iodine for export is packed in small wooden kegs of 70-kg capacity.
Coke By-Product Nitrogen
Second in the historical development of our present nitrogen industry—and second only to atmospheric nitrogen in potential supply—is the recovery of the nitrogen present in combined form in coal, shale and similar organic matter. The earth’s coil deposits, estimated at nearly 3500 billion metric tons, contain some 40 billion tons of pure nitrogen and some 190 billion tons of hydrogen, which are released with the destructive distillation of coal to form coke.
Recovery of By-product Nitrogen
In the distillation of coal, the nitrogen values are distributed between the coke, the tar, and the gaseous components. In the gaseous fraction, nitrogen is present in combination with hydrogen in the form of ammonia, which may be absorbed in water to yield aqua-ammonia or in sulphuric acid to form ammonium sulphate.
At the present time, the ammonia forms one of the most profitable by-products produced in coke and gas plants and in many cases the yield from sales of ammonia liquor or ammonium sulphate is essential to the financial stability of the coke-gas companies.
Prewar production of by-product sulphate in the United States ranged from 339,000 short tons in 1933 to 718,000 tons in 1940, with an average of 529,000 tons, according to the Bureau of Mines. Production for 1947 was reported as 820,520 tons, the increase due principally to the increased demands of the steel industry for coke.