Table of Contents
The results of column leaching tests on 3 different mixed oxide/sulfide ores from a Chilean deposit are presented. The 3 samples contained different ratios of oxide copper minerals to chalcocite and chalcopyrite. The results of the column leach tests are interpreted in light of the shrinking core model for coarse particle leaching. The copper recovery from the oxide minerals and the sulfide minerals each conforms to the shrinking core model with the total recovery equal to the weighted average of the individual minerals.
The heap leaching process is complex involving several separate physical and chemical steps taking place in parallel or series starting with the application of a leach solution to the top of a heap and ending with the exiting of this solution, now a pregnant solution, at the toe of the heap. The individual steps that make up the entire process each proceeds at a rate dictated by characteristics of the ore, the heap and the operating parameters such as heap height, solution flow rate etc.
Chemical Analysis of Oxide and Sulfide Copper Minerals
The response of the ore to leaching is dependent on the form or mineral in which the copper occurs in the ore. To determine the minerals present several analytical procedures have been developed which are referred to a “acid soluble” or “non-sulfide” copper determinations. No one procedure has been accepted as the best procedure and each procedure results in a different value for the acid soluble or non-sulfide copper content of an ore.
Which analytical procedure should be used for a given metallurgical process and a given ore deposit is a choice which will depend on the mineralogy of the ore, the process used to recover the copper from the ore and a mutually agreed upon definition of “acid soluble” or “non-sulfide” copper.
The various analytical procedures which are used to determine acid soluble or non-sulfide copper are based on the solubility of the different copper minerals to different livixiants. Most of the procedures are based on the fact that copper sulfide minerals need an oxidizing agent to solubilize the copper while (with two exceptions) the non- sulfide copper minerals are solulibized by a simple acid/base reaction.
Bacteria can play a part in the electron transfer but it is important to note that they act essentially as a catalyst in the reaction not a reactant. As such bacteria must have access to oxygen. Therefore, the overall chemical reaction is independent of the presence of bacteria. The rate of the bacteria catalyzed step in the reaction is incorporated into the overall process rate just as the rate of any other step is incorporated into the overall rate. Non-sulfide copper minerals release copper into solution by the following reactions:
Chrysocolla:
CuSiO3·2H2O + 2H+ → Cu+² + SiO2 + H2O
Malachite:
Cu2CO3(OH)2 + 4H+ → 2Cu+² + CO2(g) + 2H2O
Azurite:
Cu3(CO3)2(OH)2 + 6H+ → 3Cu+² + 2CO2 (g) + 4H2O
Of these minerals copper is (from a thermodynamic point) 100 percent released from chrysocolla, malachite, azurite and tenorite. Cuprite and native copper will not release any significant copper into solution without the addition of an oxidizing agent. Analytical procedure,
- Dissolution in a weak acid (5 percent acetic acid),
- Dissolution in a strong acid (10 percent sulfuric acid),
- Dissolution in a 10 percent solution of sodium cyanide and
- Dissolution by the standard analytical take-up (hydrochloric and nitric acid).
The Chemistry of Leaching Copper Ores
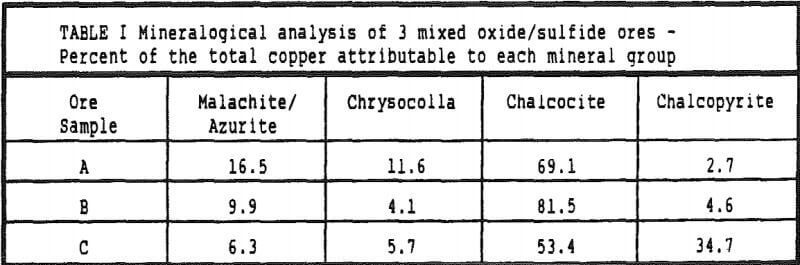
A mineralogical examination of the 3 ore types revealed a copper mineralogy which can be divided into 3 separate mineral groups: 1. the oxide copper minerals chrysocolla and malachite, 2. chalcocite and 3. chalcopyrite. Minor amounts of covellite and brochantite (copper sulfate) were also detected. In evaluating these ores for heap leaching the 3 mineral groups can be considered as distinct minerals as group 1 requires only acid to solubilize the copper, group 2 requires only ferric iron in an acidic medium and group 3 can be considered insoluble because, although chalcopyrite is soluble in ferric sulfate solution its reaction rate is so much slower than the other copper minerals that its contribution to the copper production from a heap will be low.
In addition to the oxide copper minerals being dissolved by the sulfuric acid generated by the reaction of ferric iron with the sulfides, if sulfuric acid has been added to the leach solution this acid will also dissolve copper from the oxide minerals.
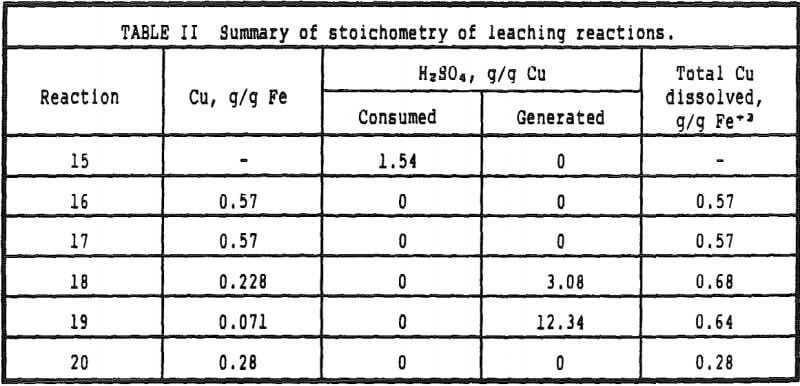
Chalcocite will dissolve completely and the sulfide sulfur will be converted to sulfate. Acid is not consumed by the gangue. This last restriction results in the calculated copper production being higher by the actual amount of other cations solubilized and the calculated reagent consumption being lower than the actual consumption for the same reason – the calculated results are therefore, a calculation of the best that can be obtained in the heap leach operation.
Modeling the Leaching of Mixed Oxide Sulfide Copper Ores
Within some restrictions the software package LEACH can be used to simulate the leaching of ores containing a mixture of oxide and sulfide copper minerals in an acid/ferric sulfate solution.
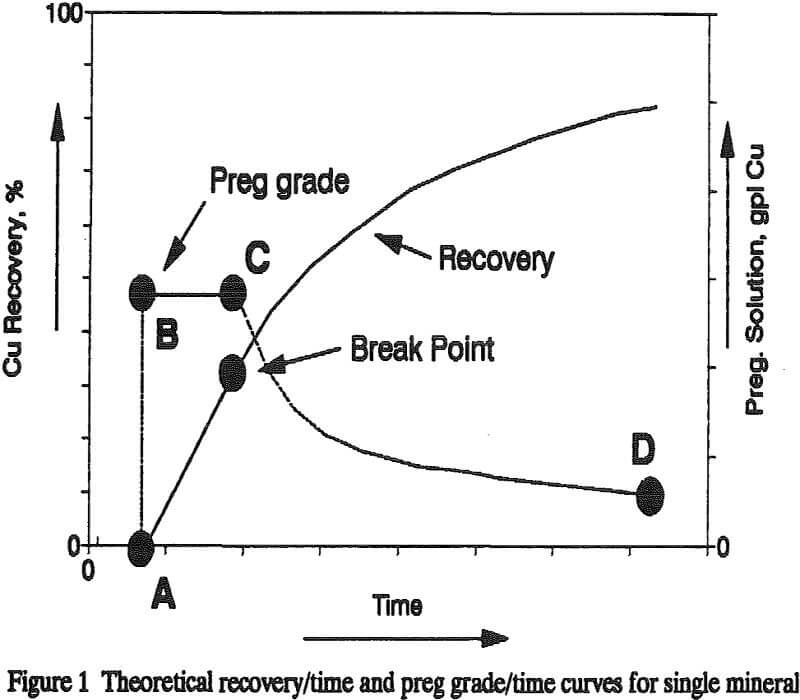
The “break point” occurs when the leached shell around a particle becomes to thick to allow all of the leaching reagent to diffuse through the leached shell during the retention time of the leach solution in the column. The point in time at which this break point occurs is a function of the ore properties and the ionic diffusivity of the reagent dissolving the copper mineral – acid in the case of oxide minerals and ferric iron in the case of chalcocite. If the oxide and sulfide minerals leach independently then the recovery/time curve for each mineral can be algebraically added to obtain a recovery/time curve for the ore.
During this period of time both the sulfuric acid and the ferric iron in the leach solution are completely consumed as the leach solution passes through the column. In the oxidation ot chalcocite by ferric iron, sulfuric acid is generated as a result of the oxidation of sulfide sulfur to sulfate sulfur. This newly generated acid is also consumed by oxide copper minerals and acid consuming gangue minerals. As a result the copper content of the preg solution is limited by the stoichiometry of the chemical reactions and the masses of acid and ferric iron added to the column per unit time.
Diffusion of ferric iron through the leached shell of the particles becomes rate controlling. Like diffusion control during acid leaching of the oxide minerals, the ferric iron concentration in the preg begins to rise. As the leach shell becomes thicker the retention time does not allow all of the ferric iron to react before the leach solution leaves the column. The copper concentration in the preg solution continues to decrease and is determined by the amount of iron which has had time to diffuse across the leached shell and the stoichiometry of the ferric iron/copper sulfide reaction.
The leaching of a mixed oxide/sulfide copper ore can be described by the addition of an oxide copper recovery curve and a sulfide copper recovery curve if certain conditions are met. These conditions include that the concentration of acid and ferric iron in the leach solution be such that the percentage recovery of copper from the oxide copper minerals in the ore must at all times greater than the percentage recovery of copper from the sulfide minerals.