Table of Contents
The program of study of pellet binder materials was begun by the ORF in 1963 to examine various Canadian materials as substitutes for bentonite which is imported in large tonnages (199,270 tons in 1969) from the U.S.A. for Canadian pellet plant operations. Most of the raw bentonite used in Canada originates in Wyoming, North Dakota and more recently Greece, resulting in relatively high transportation costs to pellet producers. Some Canadian bentonites available in Western Canada (Alberta) can be beneficiated for uses as pellet binders but could not compete In cost with U.S. sources due to the cost of beneficiation and the higher rail transport charges to Eastern Canada.
Research Program
The iron ore concentrates subjected to pelletizing tests represent four different Canadian sources. Extensive test work was carried out on three samples from the Adams Mine near Kirkland Lake, Ontario.
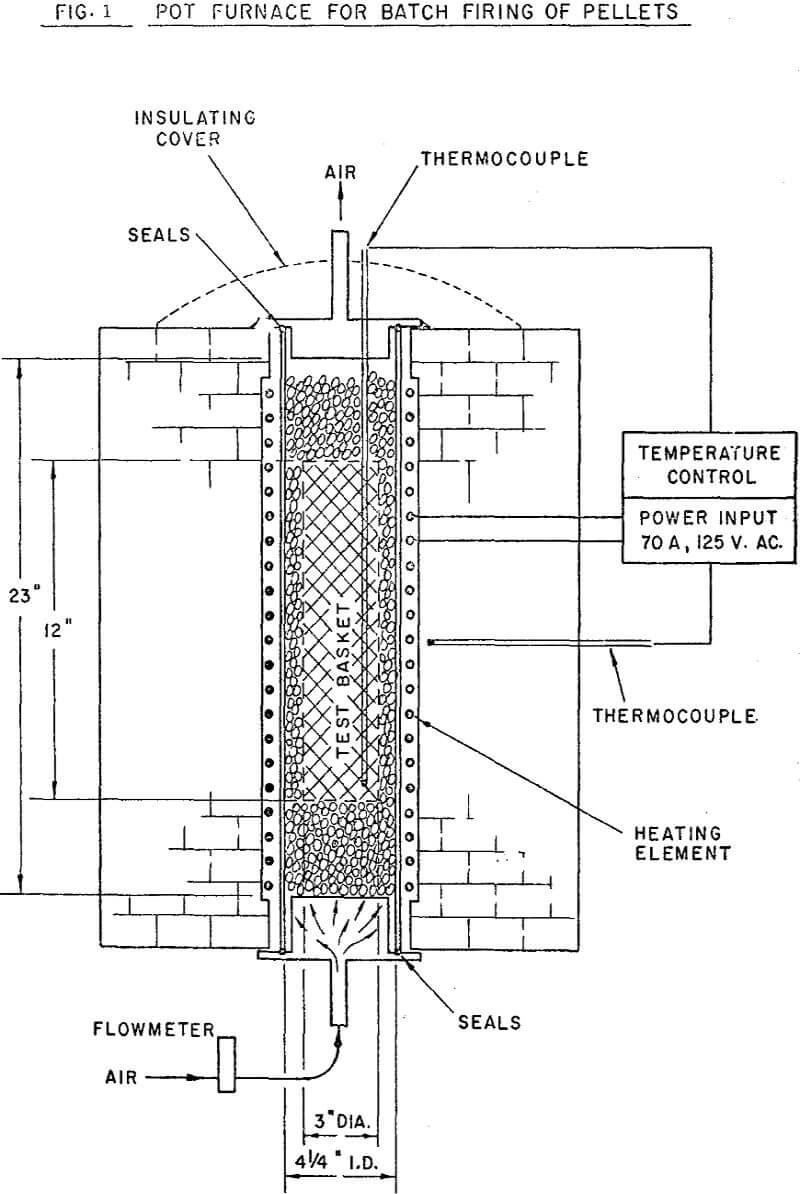
The major proportion of the equipment used in batch balling and pellet evaluation is quite common to most well equipped mineral dressing laboratories. As is the case with most extensive programs, some modification of equipment and procedures was adopted from year to year. The results presented in the current paper were, however, selected to eliminate the effect of successive equipment modifications. The batch balling, firing and pellet evaluation techniques used are also considered standard;in industry as well as research laboratories.
3000 grams of dry concentrate was mixed with 330 ml. of water in the mixing drum. The amount of material balled was determined by the quantity required for firing in the pot furnace. Binder was added to the wet concentrate in pre-determined amounts and blended into the wet mixture. The mix was passed through the shredder to break up occasional lumps and clusters.
The normal firing cycle at ORF consists of heating the charge to 1800°F for a one hour pre-heat cycle, then further raising the temperature to 2300°F. The charge is held at 2300°F for one hour, then furnace cooled overnight. During firing, an updraft air flow of 26 cuft/hr/lb of charge is maintained through the bed.
Major Variables
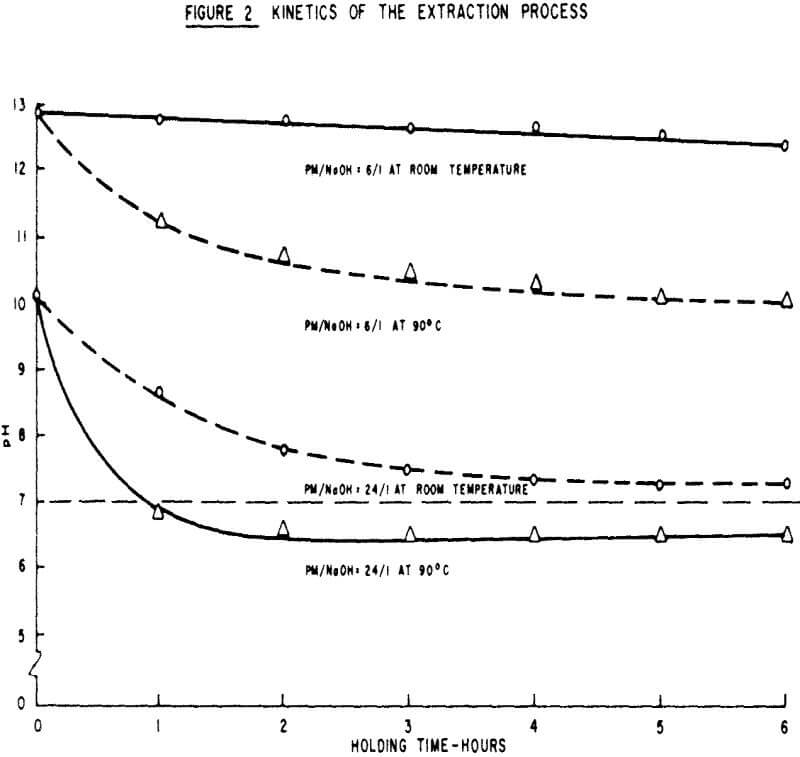
The variables affecting binder preparation were studied through batch pelletizing tests of Adams Mine Concentrates. These tests were designed to facilitate the examination of the effects of the peat moss to sodium hydroxide ratio, time of contact and temperature.
The test procedures for the extraction factorial were as follows: 40 gms of unground peat moss, 700 ml of water and the desired amount of sodium hydroxide were mixed in a 2 litre reaction kettle. In cases where the test conditions required 6 hours contact time and high temperature, the container was placed in a constant temperature bath. Following evaporation of the water in an oven (approximately 90°C), the dry residue was ground and used for pelletizing in a way similar to standard bentonite addition.
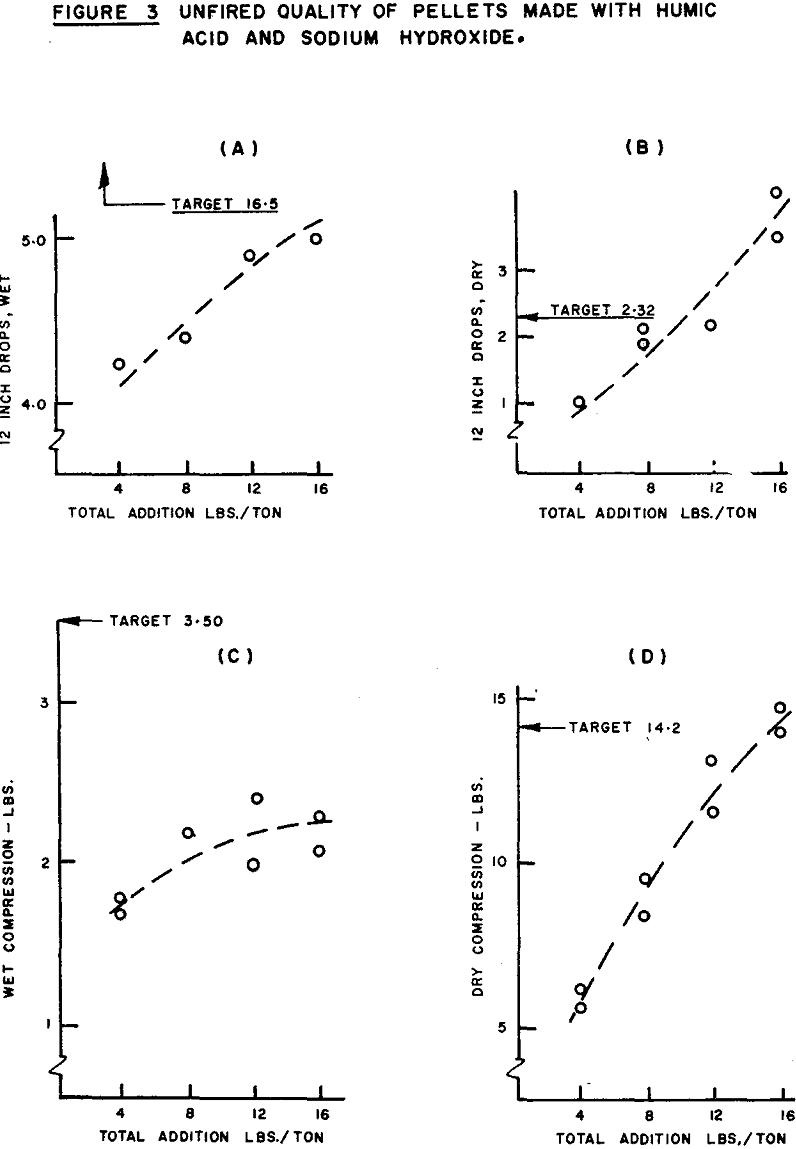
Some caution regarding the interpretation of the F ratio shown is called for upon re-examination of the pelletizing results. The higher moisture content of the pellets made with the higher level of NaOH addition amplifies the importance of NaOH. Since wet drop strength increases with moisture, the bias results in a stronger correlation (pearsonian coefficient = +0.92) between NaOH addition and green drop number than would be obtained if all tests were at the same moisture level. After the removal of the moisture bias, the coefficient of partial correlation is +0.78, indicating that a significant portion of the effect appearing to be caused by the increase in sodium hydroxide addition is, in fact, due to the increase in pellet moisture.
Applications of Peat Moss Binder
The relatively large volume of data produced for pelletizing Adams Mine concentrate samples with a variety of binders during our program, and the proximity of the peat moss deposit to this property, made the choice of this material an obvious one when the detailed evaluation of extracted peat moss was considered.
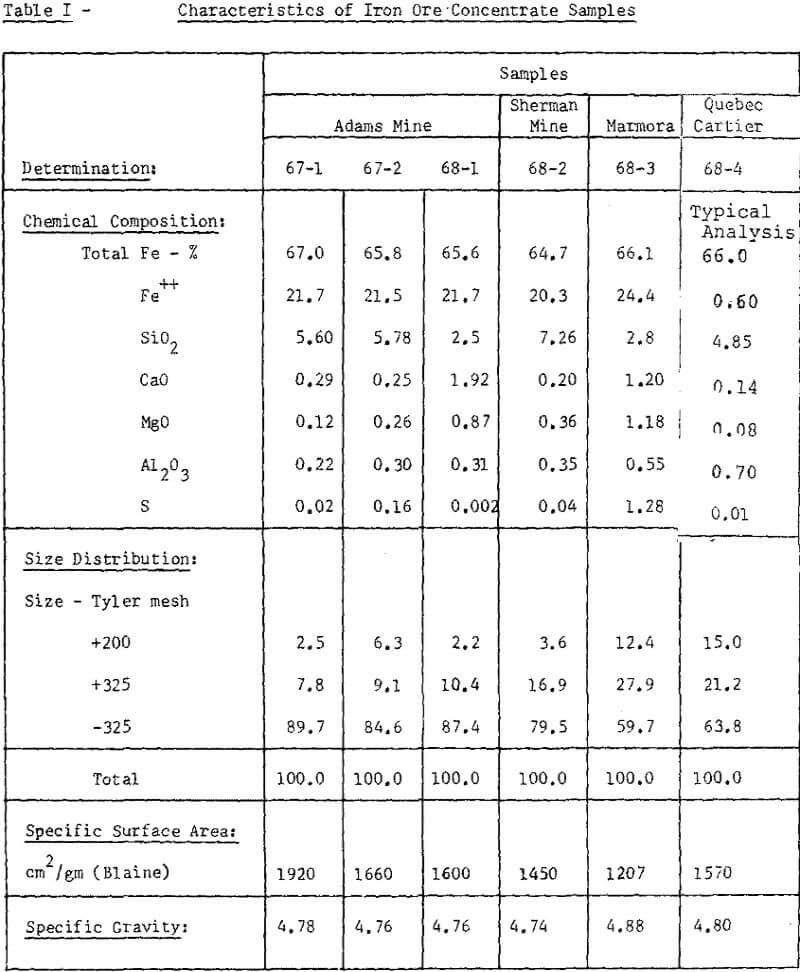
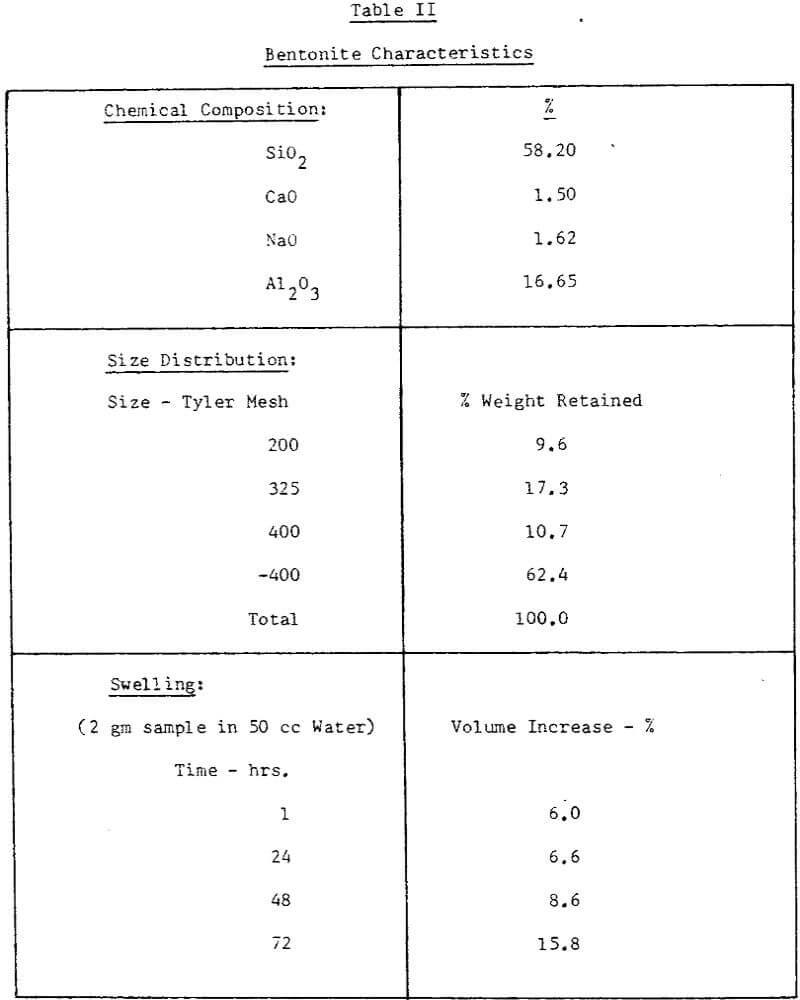
The concentrate at this plant is obtained by wet magnetic separation of a finely disseminated magnetite bearing ore. The characteristics of concentrate samples used for our testwork were presented in Table I.
Another important feature of the use of peat moss as binder is that it does not dilute the pellet with unwanted silica. Most of it burns and helps in the firing process and, after burning, it results in a porous pellet that is more easily reduced in the blast furnace.
Results of a Linder test on pellets made with 16 lbs/ton of peat moss and 1.0 lb/ton NaOH (Test No. 68-38P) also show acceptable pellet quality. The Linder Index of 4.13% minus 14 mesh and the weight loss of 9.2% during the test is considered well within the limits set for good pellets. Unfortunately, it is not possible to compare these results with similar data on pellets made with bentonite in the laboratory because this particular Linder test was spoiled. Linder test results on commercial pellets were 1.57% minus 14 mesh and 10.6% weight loss.
General Application
The pelletizing tests conducted on four different iron ore concentrates were intended to provide a wider base for the comparison of the quality of pellets made with extracted peat moss and the conventional method of using bentonite as a binder. The generation of the relevant data for these comparisons was planned in two stages, as follows:
- the establishment of control levels of quality on the basis of repeated testing of conventional bentonite-bonded pellets,
- the generation of data on pelletizing the same concentrate samples with established addition levels of extracted peat moss. The binder addition used in each case was established in previous tests as 16 lbs/ton of peat moss extracted with 2 lbs/ton of sodium hydroxide.