Table of Contents
Electrostatic separation is used when froth flotation fails, such as with oxidized ores, or where there are similarities in response with magnetic and gravitational methods, or when the use of water is to be avoided. Theoretically, for fine-size particles the electric field has an advantage over the magnetic field in that the viscosity-limited particle velocity varies with the first power instead of the second power of the particle diameter. However, electrostatic separation has been confined to sand-size particles, which are required when rotating-roll and inclined-plate separators are used. Particles coarser than 200 mesh (74µ) may be considered “free flowing”; particles finer than this tend to form agglomerate masses that remain undispersed when passed over the rolls and plates. MacGregor in 1914 stated that the increased occurrence of finely disseminated ores contributed to the discontinuance of the electrostatic method. The fine particles not only remained unseparated, but, as described in the discussion included in the reference of MacGregor contributed to a dust hazard and nuisance factor. There is not only a need for the electrostatic separation of the small particles in finely disseminated ores, such as nickel and copper laterites, but also for the fine-size fraction that is produced from ores that require only coarse-size crushing to unlock the minerals. Except for some early work by the Bureau of Mines, whereby contact electrification was accomplished by passing air suspended particles through a jet, the electrostatic separation of fine-size particles has never before, been attempted. Photoelectric charge production provides a new approach.
Photoconductivity Versus Photoemission of Electrons
The photoelectric property of minerals has been described in reference 7. Randall and Wilkins report a photoconductive effect in air for scheelite (CaWO4) that is more pronounced at 3,650 A than at 2,560 A, and refer to Hofstadler and Herman, who found a photoconductive effect for willemite (Zn2SiO4) at the shorter wavelength. Although the photoelectric yield was quite low. Hughes. Tredgold, and Williams found that the photoemission of electrons from barium titanate (BaTiO3) decreased upon exposure to air, having a value of 2.5 x 10 -4 electrons per photon at 2, 000 A, a value which increased rapidly with decreasing wavelength. Apker and Taft found no photoemission of electrons from films of potassium iodide (KI), but that an emission was later obtained at 2, 200 A when F centers were formed on continued irradiation.
For electrostatic separation of fine powders, a photoconductive effect is of little value because it requires contact of each particle with an electrode surface. In contrast, the photoemission of electrons occurs in free space and a large number of particles may be charged in a single exposure. Direct application of the photoelectric effect to mineral separation has been demonstrated with sphalerite (ZnS). The additional positive electrode data presented in the present report will show that the effect is due to the photoemission of electrons.
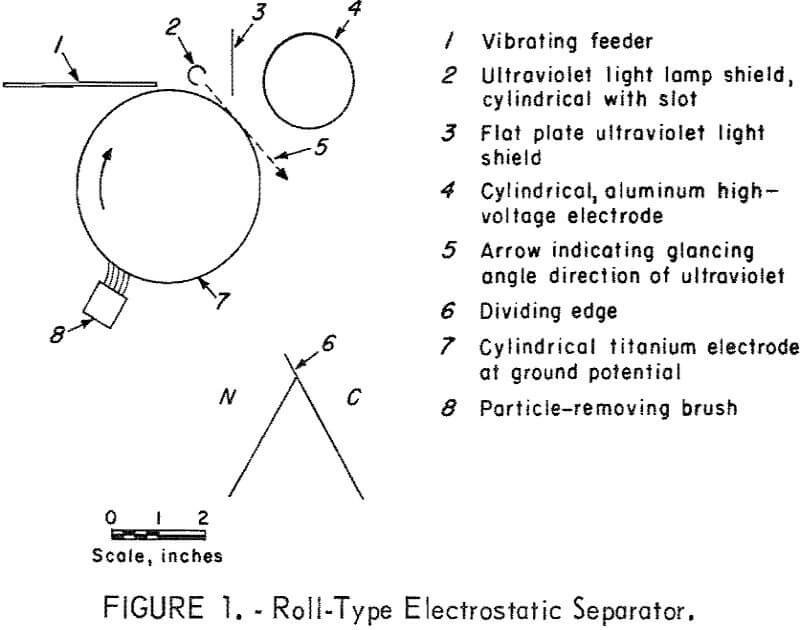
The separator (fig. 1) is the simple static field roll type except for the titanium metal cylinder and the mercury vapor lamp. This lamp is constructed of 0.25-inch-outside-diameter, double-capillary quartz tubing sealed at one end so that the two capillaries form a U-tube with two legs, which are each fitted with electrodes. The lamp shield directs the rays so that they pass the cylinder tangentially. This orientation of the radiation minimizes the photoemission of electrons from the titanium and also provides illumination of the particles at a point close to the cylinder to permit more effective use of any photoconductivity that may occur. Titanium was selected for the cylinder because of its high photoelectric threshold. The high-voltage electrode, which has a potential of 30 kilovolts, either attracts or repels the particles.
The vibrating feeder was constructed of aluminum, and, from reported data, this would result in the sphalerite being charged negatively by contact electrification. The contact electrification accounts for the results of table 1 “without ultraviolet radiation.” A positive high-voltage electrode attracts the negatively charged sphalerite providing for a larger quantity being collected at point C than when the electrode is negative. With ultraviolet light there is an emission of electrons leaving the sphalerite positively charged with the result that a greater amount of feed is collected at point C when the high-voltage electrode is negatively charged. That the effect is photoemission and not photoconductive is shown by the small amount of material deflected, 2.1 percent, when the high-voltage electrode is positive. If a photoconductive effect occurred, the amount deflected would be large with either a positive or negative electrode, since the increase of electrons and holes by photons provides for passage of a current in either direction.
Conductive minerals, which would always have a maximum collected at point C, cannot be tested for photoemission with this apparatus. A large number of nonconductive minerals were tried, but only sphalerite was found to respond. Sensitivity to response could be increased with a higher intensity lamp or by the use of shorter wavelengths with the subsequent increase in photoelectric efficiency, although this would involve the problem of ultraviolet light absorption in air.
Ultraviolet Light Absorption in Air
At shorter wavelengths, ultraviolet light absorption in the form of bands and continua occurs in air. For O2 it extends from 2,000 A down to 300 A. Bands for O2 extend from 2,000 1 to 1,750 & with 14 peaks having maximum values of a, which range from less than 0.1 at 2,000 A to 0.1 at 1,850 A, and less than 10 at 1,750 A. The continua for O2 and other gases are summarized in table 2.
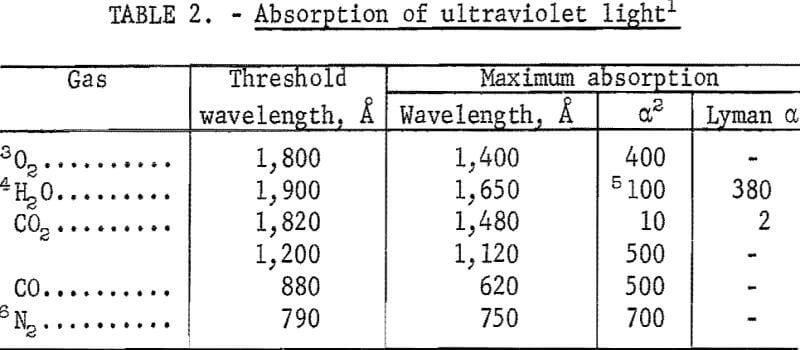
Nitrogen is transparent down to 1,120 A. With oxygen and nitrogen mixtures corresponding to air, transparent windows are located at and near the Lyman α line of 1,216 A. Of the various combinations of gas composition and wavelength that may be selected for transparency, H2O is the primary obstacle. Table 2 shows a value of α = 15 for this gas in the range of 1,430 to 1,450 A, which may be a good operating region for N2 and CO mixtures relatively low in H2O. Windows of MgF2, which are conveniently sealed with viton O-rings, can be used at this wavelength. However, a more practical combination appears to be air in the range of 1,800 to 2,500 A, using an ultra violet lamp that has its spectral output concentrated in this range. The deuterium continuum, is most ideal for this purpose, since the spectral distribution curve has a maximum value near 2,000 A. It is superior to the output of the mercury vapor lamp, whose discontinuous spectral curve drops close to zero at 2,000 A.
Photoemission Effects of Conductive Minerals
A simple method for the photoemission detection of all minerals, including both conductors and nonconductors, utilizes the fiber suspension apparatus illustrated in figure 2. A relatively large particle was selected to minimize any photoelectric effects of the fiber. As illustrated in figure 2, the particle is suspended above the center line through the positive and negative spheres to provide for a more stable deflection. Also different from the data of table 1 is the use of a deuterium lamp, which has a high-purity quartz window that provides 70-percent transmission at 1,750 A. This is a point source arc type with a filament used only for starting. Initial exploratory tests showed deflections in the order of decreasing magnitude for the solids: filter paper, aluminum metal, and sphalerite. Little if any deflection was obtained with plexiglass sheet, bauxite, pollucite, and brucite.
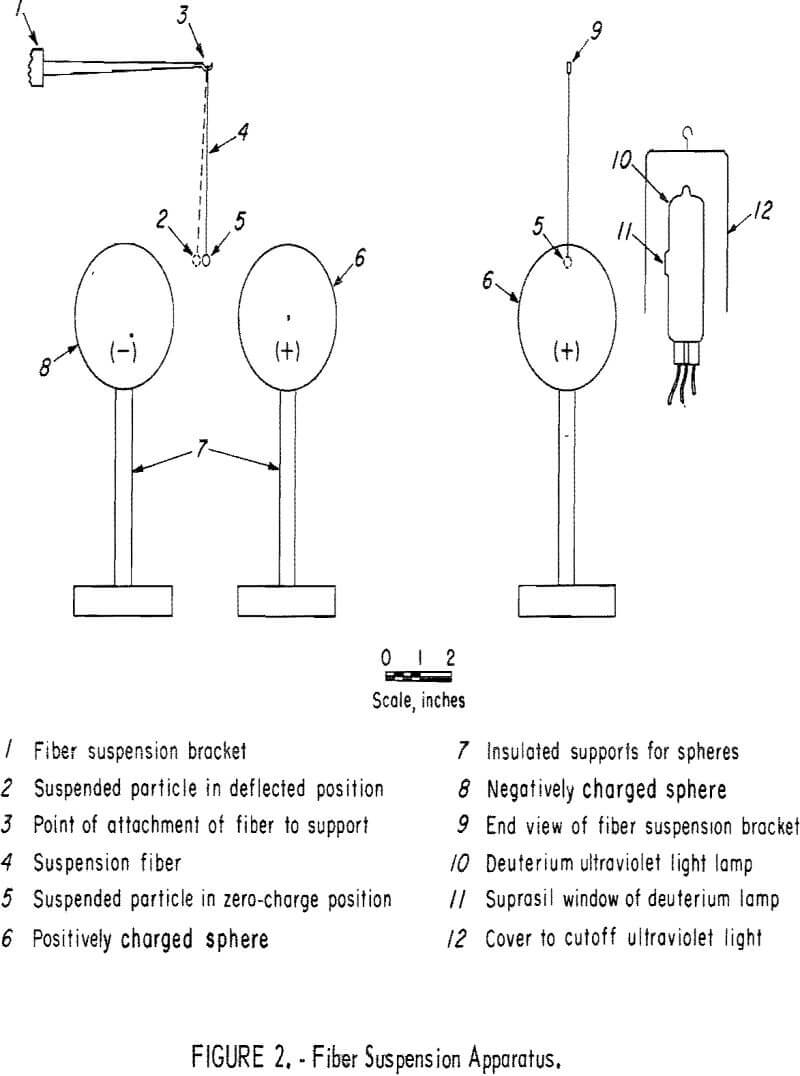
Table 3 summarizes more precise measurements for several typical minerals providing the radiation time t to obtain a 0.25-inch horizontal deflection. A 1-minute warmup period is required for the ultraviolet lamp, which finally operates at 60 watts; the sample irradiation time is the interval during which the cover is lifted away from the lamp. The potential supplied to the positive and negative electrodes was grounded at midpoint. Once the particle acquired a charge by photoemission of electrons it could be brought back to zero deflection by touching with a ground-potential conductor. Figure 3 shows the gravitational and electrical forces fg and fe perpendicular to each other and the fiber suspension force fs. When the particle is deflected an angle θ with the vertical,
fe/fg = tan θ……………………………………………………………….(1)
where θ may be determined from the fiber length, ss, and the deflection distance Se,
Se/Ss = sin θ…………………………………………………………….(2)

For a 0.25-inch (0.64-centimeter) deflection and a 10-centimeter fiber length, θ = 3° 40′.
Introducing the expression for the electrical and gravitational forces, where q is the charge density over the projected area A of an irradiated particle of volume V,
fe = AqE……………………………………………………………(3)
and
fg = Vpg……………………………………………………………(4)
or
AqE/Vpg = tan θ……………………………………………….(5)
The tests were conducted with 30 kilovolts across the spheres with the particle center 1 inch above the center line through the spheres, at which point the field intensity estimated graphically by the method of curvilinear squares, is 16 statvolts per centimeter. Introducing E, θ, and g in equation 5,
q = 3.92 pV/A………………………………………………………..(6)
Since the particles, except for rutile, were blocky approximations of spheres and the data provide only values for m and p, calculations for q and p are based on a spherical shape,
V/A = 4/3 r……………………………………………………….(7)
The value for r may be found by equating the mass to the volume and density
r = (3m/4πp)1/3………………………………………………….(8)
Combining equations 6-8,
![]()
The values for the photoelectric charging rate p are obtained by using the equation,
p = q/t……………………………………………………(10)
Rutile is calculated by using equations 3 and 4 on the basis of a parallelepiped with the radiation received by one of the 0.24- by 1-centimeter faces.
Photoemission from Dust-Size Particles
With dust-size particles, where the particles are suspended in air, additional factors must be considered. One of these is the formation of clusters resulting from the attractive forces between oppositely charged particles and the coagulation effect of intense electric fields. For this reason the apparatus of figure 4 was designed around a sonic-dispersion system that involves the use of two vibration drive units, electrically connected opposite so that the diaphragms move in the same direction at the same time. This phase relation, which was adapted from Withey and Van Dyk, who used it with direct radiator-type acoustic speakers for imparting a pumping action to sieves, results in the air between the drivers moving back and forth as a single body providing a dispersing effect on dusts. It is in direct contrast to the application of sound for the precipitation of dust, where one sound source is used although two traveling waves occur, one moving from the source and a reflected wave moving from the opposite closed end of the air column. Nodes and antinodes are formed, and since the radiation pressure drives the particles to each antinode from each of its sides, there is an accumulation of a large number of particles in a small volume with subsequent coagulation and precipitation.
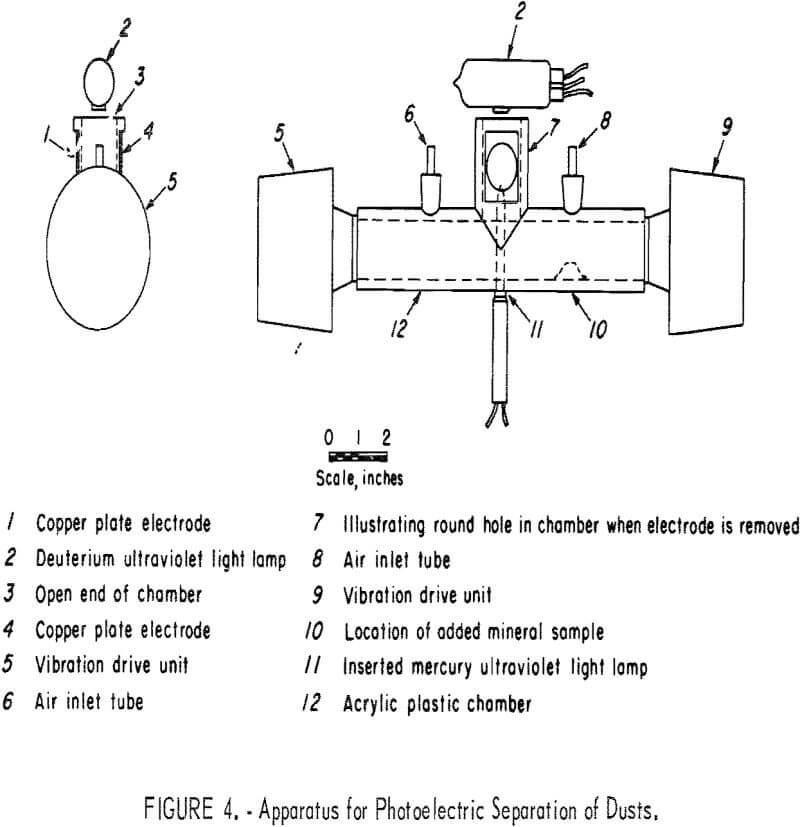
In figure 4 the column of moving air is contained in the chamber, which is constructed of 1.25-inch-inside-diameter by 1.75-inch-outside-diameter acrylic plastic tubing with a vertical section cemented at the center to form a tee, which has two copper plate electrodes covering round holes in the chamber so as to form an electric field space. Ultraviolet light can be provided by the deuterium lamp (also shown in table 3), or the mercury vapor lamp (also shown in table 1); the mineral sample (dust) is introduced through the air inlet tube. The drive units, University Sound 1D-20, are rated at 20 watts and 16 ohms and are powered with an MB Electronics model 2250 MB power amplifier and a General Radio 1310-B variable-frequency oscillator. The drivers have sufficient power to disperse the pile of dust into an air suspension. However, some of the dust is trapped in the drivers and not recovered. This could be corrected with the drivers turned 90° so that the apertures face downward and are connected to the chamber with 90° ells. A frequency of 610 to 630 hertz appeared to give the best results.
In the tests summarized in table 4, the mineral sample was added through an air inlet tube, the deuterium lamp was permitted to warm up to 60 watts, and finally the acoustic drivers were started. During this procedure the total measured air velocity was divided between the two air inlets. Upon operation of the acoustic drivers the dust started to collect on the electrodes and after 30 seconds the electrodes were removed for recovery and weighing of the collected dust. Because the acoustic drivers were the primary movers of the dust, the dust could be collected even without an air stream; but with only the air stream no dust was collected.
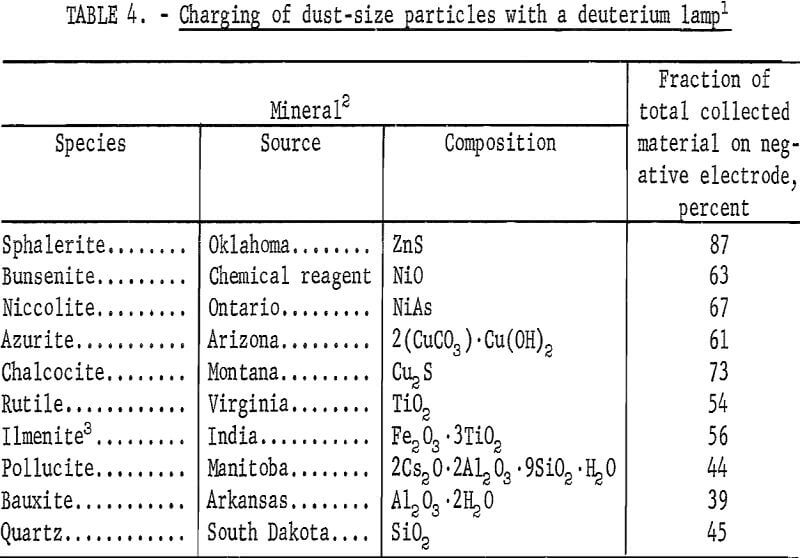
The characteristic of the charging produced by the dispersion of powders from a bulk mass to a suspension in air is the occurrence of equal amounts of positive and negative particles. Visual observation indicated that this was true for quartz, bauxite, and pollucite when the minerals were first ground, but approximately 1 month later there appeared to be a greater proportion on the positive electrode. Actually, the whole spectrum of photoelectric and nonphotoelectric minerals had shifted in a negative direction. Table 4 summarizes the quantitative measurements taken at this later time. The order of photoelectric response is in agreement with that of table 3, except for sphalerite in table 4; there this mineral is much higher than bunsenite, although the relative high value for the bunsenite pellet in table 3 may be attributed to the larger surface area resulting from the pellet assembly of small particles. The same mercury vapor lamp used in table 1 is less effective in producing photoelectric charging (as illustrated in table 5).
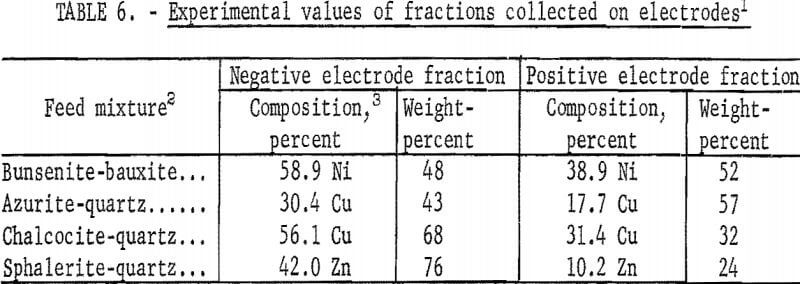
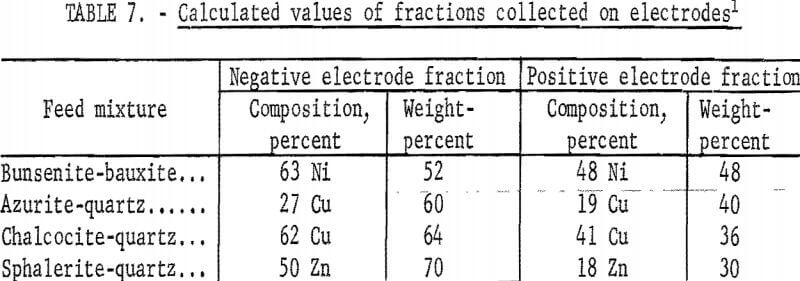
When mixtures instead of pure minerals are used in the apparatus in figure 4, the results of table 6 are obtained. These experimental values, when compared with the calculated values of table 7, are found to agree within the limits of experimental error. Such an agreement would not be possible if coagulation and clusters of positive and negative particles occurred, since cluster formation with mineral mixtures would provide results that would be in variance with the results obtained with cluster formation with the pure mineral. It is therefore evident that coagulation and cluster formation is not a problem and that the sonic method provides for complete dispersion.
Charge Required for Dust Separation
For a spherical particle, the Stoke’s force of resistance is represented by the equation,
fv = 6πηr ds/dt…………………………………………………………..(11)
which is in equilibrium with the electrical force on the particle, or,
fe = πr²ptE……………………………………………………….(12)
where the charge area is the projected area of a sphere that receives radiation from a fixed direction at rate p. If the particle has received its photoelectric charge q before entering the electric traction field, equation 12 changes to,
fe = πr²qE……………………………………………………….(13)
Combining equations 11 and 12 and integrating
s = rpE/12 t²…………………………………………………..(14)
with equations 11 and 13,
s = rqE/6η t…………………………………………………..(15)
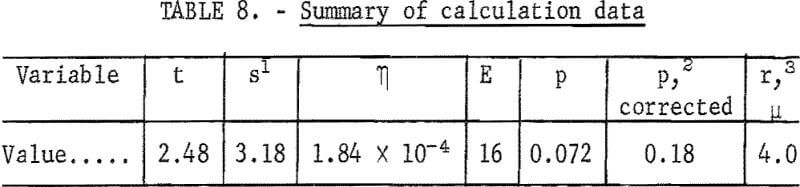
Time t may be estimated from the air velocity of 8 cubic centimeters per second, the chamber cross-sectional area of 7.92 square centimeters, and the electrode diameter of 2.5 centimeters. This is the time for air flow across the electrode diameter. Other values are listed in table 8.
By this calculation, which is based on a maximum field direction travel and an average ultraviolet light intensity corresponding to the arc point to electrode center distance, initially uncharged particles having a diameter greater than 8µ, and the azurite-charging rate (table 3) should be collected on the negative electrode. Particles smaller than 8µ would pass beyond the electrodes, but still could be collected by a change in electrode design to provide for a smaller s/t² value.
In a comparison with the azurite data of table 4, the 61 percent represents a fraction of the collected material, the remaining 39 percent is residual azurite, which still retain a negative charge. Accordingly, some factors have not been considered. One of these is interfering charges. If the charges generated by the dispersion of powders to provide equal quantities of positive and negative charges are much greater in magnitude than the photoelectric charge, the photoelectric charge will not have much effect in altering the charge distribution. Other factors are the screening of radiation, or the shadows produced by particles closer to the lamp, interference of positive and negative particles moving in opposite directions, particles outside the main ray zone, and the neutralization of the positive photoelectric charges by the photoelectric electrons emitted from the irradiated electrodes. There, is also the problem of the disposal of the photoelectrons emitted from the irradiated minerals. Direct passage to the positive electrode has been considered, although recombination with the mineral particles may occur under certain conditions.
It is quite probable that the only, or at least the most significant, interference factor is the large value of the positive and negative charges produced by the dispersion of the powder. Neutralization preliminary to photoelectric charging would permit all of the particles to be photoelectrically charged positive on the basis of table 8, or preferably, neutralization followed by the addition of a very small negative charge preliminary to photoelectric charging would provide close to complete separation of the mineral mixtures of table 6. Neutralization of highly charged particles has been accomplished by Whitby and Peterson by adding a mixture of small positive and negative ions to the air suspension, either by means of ionizing radiation or by a stream of ion-carrying gas.
Conclusions
Although complete separation of mineral mixtures was not attained in one stage, it is probable that this may be improved by preliminary neutralization of the initial electric charges produced by powder dispersion. An important factor is that the separations can be carried out in air of average humidity without the use of reagents or heating. There are further possibilities for improvement in ultraviolet lamps, particularly in lamps having a maximum in the spectral distribution curve near 2,000 A. The deuterium lamp is a recent development in this direction. Lamps that are now available are of a small size for spectrometer use and not demountable for the servicing required in long, continuous operation.
