Table of Contents
There has been considerable interest in developing methods to economically produce cell-grade alumina from nonbauxitic materials. In the United States, this interest stems principally from a lack of sufficient bauxite deposits suitable for Bayer processing. In 1973, the Bureau of Mines initiated a program to study the options available for recovering alumina from domestic resources. The research impetus was based on the national need to maintain an adequate mineral supply to meet economic and strategic goals. Because kaolinitic clays are abundant and have a relatively high alumina content, research has centered on this resource.
It was recognized many years ago that the alumina content of clay is easily soluble in mineral acid after calcining at 750° C. Of the mineral acids, hydrochloric acid (HCl) leaching offers several advantages that have prompted intensive investigation of the HCl-clay process; these advantages are (1) easy removal of iron from the aluminum chloride liquor by solvent extraction, (2) selective crystallization of AlCl3·6H2O by sparging HCl gas into the pregnant solution, (3) easy conversion of the AlCl3·6H2O to Al2O3 by fluidized-bed roasting with recovery of HCl gas for recycle, and (4) recovery of HCl from process streams at low temperature.
In addition to aluminum and iron, HCl dissolves many minor impurities from the clay. Their individual concentrations in clay are generally less than 0.1 pct, but in a continuous process they can build up to significant levels in the recycling liquors. The components of the greatest concern are Ca, Mg, K, Na, and P. Most of these impurities are effectively separated from aluminum by the AlCl3·6H2O crystallization step, which is effected by absorbing HCl gas into the aluminum chloride solution. Ca, K, Na, and residual Fe do not cocrystallize with the AlCl3·6H2O and are less than maximum specifications in the final alumina product. Phosphorus and magnesium levels are above maximum limits, and recrystallization of AlCl3·6H2O is necessary.
This paper describes a technique developed by the Bureau of Mines as an alternative to adding a recrystallization step to the HCl-clay process. The process eliminates the solvent extraction step for iron removal. The technique utilizes a strong acid-weak acid leach (SAWAL) and has the potential to make an excellent separation of AlCl3·6H2O from soluble clay impurities. The procedure is based on initial removal of the majority of soluble impurities from calcined clay with a strong acid leach; the AlCl3·6H2O is very slightly soluble in concentrated aqueous HCl. After removing the contaminated strong acid solution, AlCl3·6H2O is dissolved with a weak HCl solution. The technique can be used with calcined clay, which reacts with strong HCl. If fluoride ion is added to the strong acid leach, clay calcination can be omitted because HCl and fluoride attack uncalcined clay. The work described in this report was done with calcined clay.
Materials and Procedures
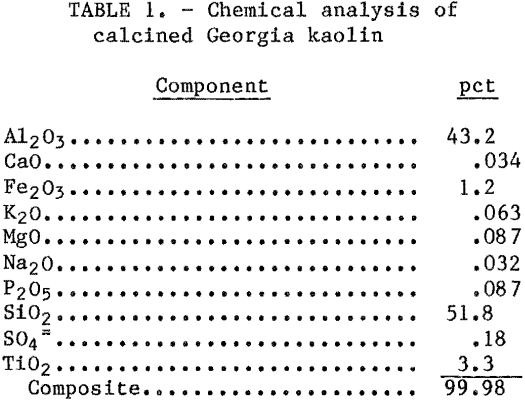
Tests were performed with a Georgia kaolin that had been calcined at 750° C and ground to minus 10 mesh. Its chemical composition is given in table 1.
Leaching solutions were made up from reagent-grade aqueous HCl and HCl gas.
Strong-acid leaching tests were performed by contacting 600-g samples of the calcined clay with 1,800 mL of aqueous 36-pct HCl and periodically injecting HCl gas into the slurry. The temperature of
the slurry was maintained at 105° C for 2 h to complete the reaction. After cooling to room temperatures acid concentration was determined by titration, and gas injection was resumed until approximately 35-pct-HCl concentration was obtained. Ideally, strong acid leaching would be conducted in a closed system so that acid concentration could be maintained at leaching temperature. Since this equipment was not available, the net effect of cooling and reacidification was probably to increase the impurity level in the AlCl3·6H2O crystals. Since time did not permit operation of the leaching system to attain steady-state conditions before bleedstream removal, the composition of the leaching liquor at a steady state was obtained by calculation. This was done for each component by dividing the concentration of the component in one-pass leaching liquor by the size of the bleedstream expression as a fraction of the whole stream. For example, if component A had a concentration of 0.6 g/L in the one-pass leaching liquor and a bleed- stream of 20 pct was taken, the concentration of component A in the leaching liquor at a steady state would be 0.6/0.2 = 3 g/L. Of course, solubility limits cannot be exceeded.
After filtration of the liquor containing the dissolved impurities, the residue was washed once by displacement and twice by repulping with 1,200-mL portions of 36-pct HCl. Weak-acid-leaching tests were performed by contacting the washed residue for several hours in a reactor fitted with a reflux condenser and containing 300 mL of boiling recycled 26- pct-HCl mother liquor from the subsequent sparging step. The silica tailing was separated by filtration and displacement washed with three 600~mL portions of hot water to remove entrained aluminum chloride.
Crystallization studies were made in a 4-L bench-scale draft tube crystallizer. The crystallizer was initially evaluated with an aluminum chloride solution of the same composition as a solution that had been used for crystallization studies in a 350-gal crystallizer. AlCl3·6H2O crystals from both crystallizers had the same purity and size distribution. HC1 gas injected into the crystallizer was diluted with nitrogen to insure even dispersion and slower injection of HCl into the feed solution. Sparging continued until the HCl concentration was 26 pct. Conditions employed were slurry temperature of 60° C, N2 flow of 250 mL/min at a pressure of 20 psig, HCl flow of 1,300 mL/min at a pressure of 13 psig, and stirrer speed of 600 rpm to draw the slurry-HCl gas mixture through the center and up the outside of the draft tube. At the conclusion of sparging, the crystal slurry was separated on a fritted glass filter. The crystals were displacement washed once with 500 mL of concentrated HCl and reslurry washed twice with 1,000-mL portions of concentrated HCl. The crystals were dried for 8 h at 80° to 95° C.
Aluminum analyses were performed by atomic adsorption and by a wet-chemical method. P2O5 analyses were made by a spectrophotometry method. Analyses for Fe2O3 , MgO, CaO, Na2O, and K2O were done by atomic absorption. Although results of crystallization tests are reported in terms of percent impurity in Al2O3, analyses were made on AlCl3·6H2O crystals and the results were converted to the equivalent in alumina.
Results and Discussion
Initial reaction between the calcined clay and HCl took place in a stirred reactor. HCl gas was added to the leaching system to maintain the HCl concentration at saturation. Table 2 shows that P2O5, MgO, K2O, and Fe2O3 impurities in the calcined clay were very soluble in 36-pct HCl. Although separation of impurities from aluminum is excellent for all of the bleedstream sizes, when the bleedstream is small, impurity concentration in the leaching liquor is high. Consequently, more soluble impurities are retained by the residue and carried into the weak acid circuit. This is shown in table 3, which gives the composition of the leaching liquor generated in the weak acid circuit after leaching with a strong acid with different size bleedstreams.
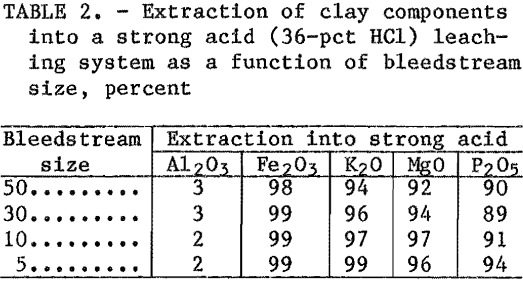
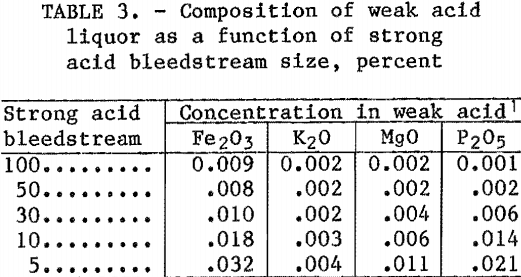
The weak acid liquors reported in table 3 are cleaner than liquors produced In the standard HCl-clay process where a 100-pct bleedstream liquor contains 0.02 pct P2O5 and 0.02 pct MgO. The values in table 3 were derived with one-pass weak acid liquor. These impurities will increase during recycle and will depend on the size of the bleedstream taken in the weak acid circuit.
Table 4 shows the P2O5 concentration of the weak acid solution for different combinations of bleedstreams in the strong acid and weak acid circuits. A tradeoff must be made between the sizes of the bleedstreams for the two circuits to arrive at a P2O5 concentration, which is acceptable in the feed to the HCl sparger-crystallizer.
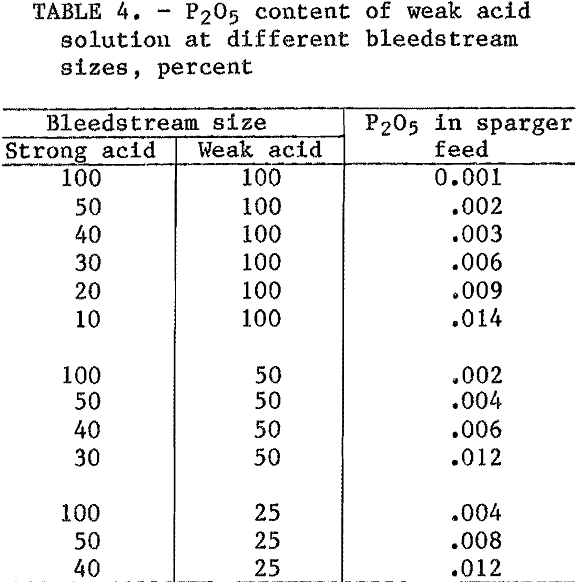
To determine the level of impurities in aluminum chloride sparger feed that can be tolerated in the production of AlCl3·6H2O of acceptable purity, the crystallization process was examined by sparging aluminum chloride liquors of widely varying P2O5 compositions. When the concentrations of P2O5 in the sparger feed were plotted against the P2O5 contents of the crystallized AlCl3·6H2O, the curve shown in figure 1 was obtained. This curve is useful because it can be used to determine whether a given liquor will produce alumina of the desired P2O5 specification. The P2O5 concentration and, to a lesser extent, the MgO concentration are limiting factors in producing
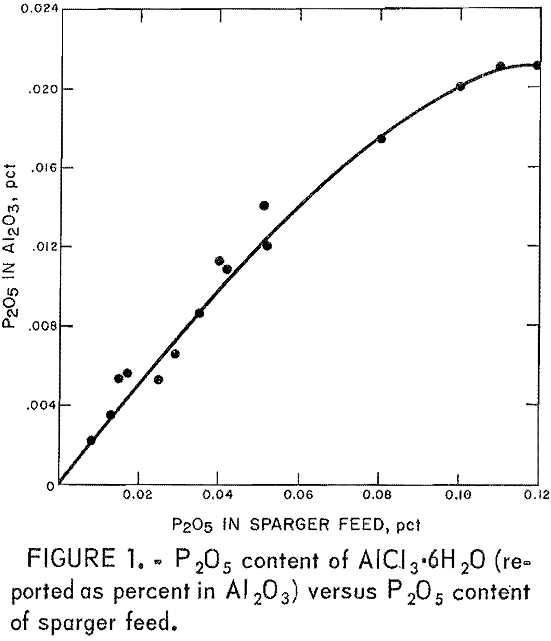
cell-grade alumina from clay. The curve shows that acceptable crystals cannot be produced from a weak acid feed liquor containing 0.01 pct P2O5 if a level of 0.003 pct P2O5 is required in the alumina product. If the alumina specification is 0.001 pct P2O5, the sparger feed must not contain more than 0.005 pct P2O5.
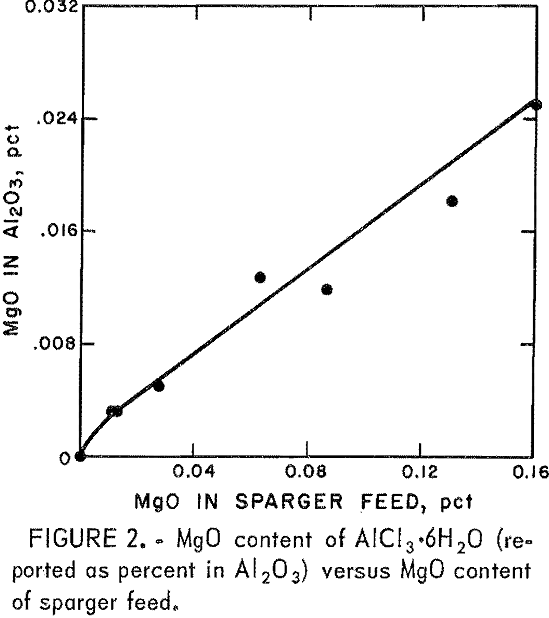
Plotting the concentration of MgO in sparger feed as a function of its concentration in AlCl3·6H2O yielded the curve shown in figure 2.
The bleedstream combinations required to produce a sparger feed that will yield acceptable alumina can be estimated from table 4. If the P2O5 specification is 0.003 pct, a number of combinations are possible. For example, a 100-pct weak acid bleedstream with a 20-pct strong acid bleedstream, a 50-pct weak acid bleedstream with a 40-pct strong acid bleedstream, or a 25-pct weak acid bleedstream with a 50-pct strong acid bleedstream could be used. If the P2O5 specification is 0.001 pct, the bleedstream sizes are greatly increased. A 40-pct strong acid bleedstream with a 100-pct weak acid bleedstream, a 50-pct strong acid bleedstream with 50-pct weak acid bleedstream, or a 100-pct strong acid bleedstream with 25-pct weak acid bleedstream are required. The same reasoning can be used with MgO content of sparger feeds. However, in all cases where the P2O5 specification is met, the MgO content will not exceed specifications.
Flowsheet
A flowsheet developed for SAWAL is shown in figure 3. Calcined clay is placed in leaching vessel (step 1), where it contacts recycled strong acid leaching liquor. HCl gas must also be injected into the slurry to maintain acid strength, otherwise some AlCl3·6H2O will dissolve and be lost in the bleedstream from the strong acid circuit. A liquid-solid separation takes place between the strong acid leaching liquor and the residue, which contains mostly silica (SiO2) and most of the aluminum as AlCl3·6H2O crystals (step 2). The residue is washed
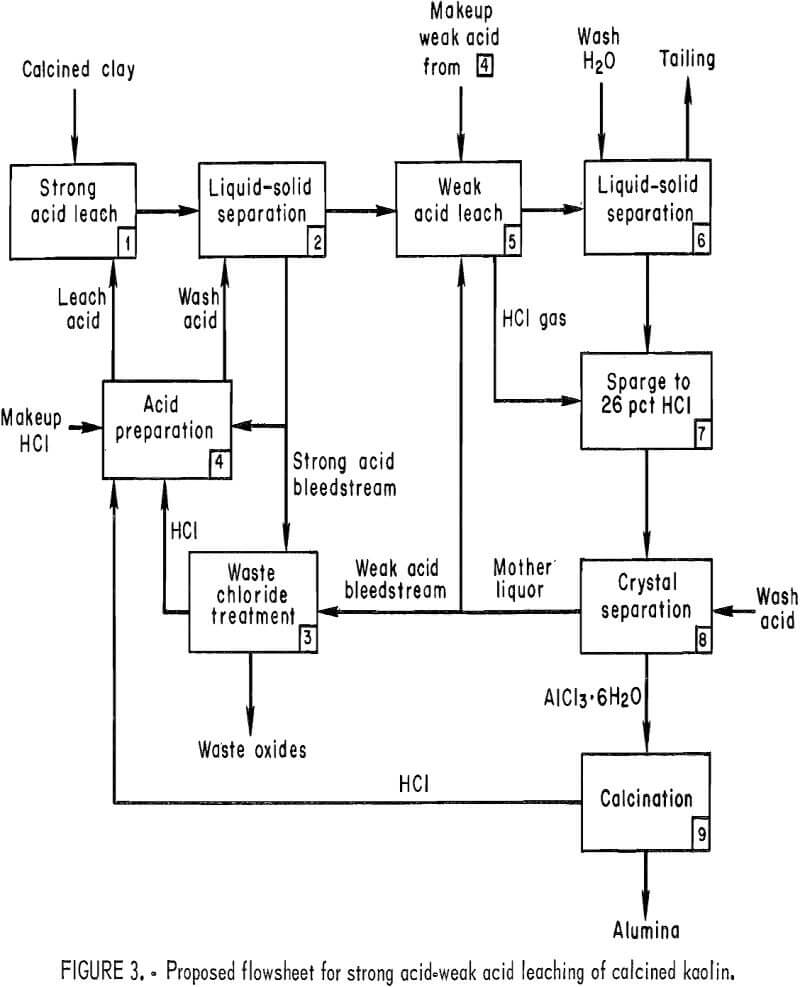
countercurrently with aqueous 36-pct HCl to remove as much of the entrained liquor as possible. After a bleedstream is taken, the strong acid liquor is sent to acid preparation (step 4) for recycle to leaching. The bleedstream is routed to waste chloride treatment (step 3), where free HCl is recovered by heating the liquor and additional HCl is reclaimed from the crystallized salts by an oxidizing roast. This step is similar to the bleedstream treatment developed for the HCl-clay process.
The residue from step 2 (AlCl3·6H2O and SiO2) enters the weak acid portion of the circuit (step 5) and is contacted with 26-pct-HCl mother liquor recycled from the sparging step. When the residue and the mother liquor are heated, HCl gas is vaporized and the AlCl3·6H2O is dissolved. Since a strong AlCl3 solution functions as an azeotrope breaker, the aqueous HCl concentration is decreased to about 10 pct, and almost all of the AlCl3·6H2O is dissolved. After appropriate washing to recover soluble aluminum (step 6), the silica tailing is separated from the AlCl3 solution and discarded. The HCl gas vaporized is returned to sparging. If desirable, the AlCl3 content of the liquor can be concentrated by evaporation before sparging. Sparging (step 7) is a reversal of step 5. HCl gas is injected into the solution until HCl concentration in the mother liquor is 26 pct. The AlCl3·6H2O crystals are separated from the mother liquor and washed with 36-pct HCl (step 8). The mother liquor from crystallization is returned to weak acid leaching after removing a bleedstream for impurity control. The washed crystals are calcined (step 9). The AlCl3·6H2O is decomposed to the final-product alumina, and HCl is sent to acid preparation. Technology developed in the standard HCl-clay process can be directly applied.
Economics
A preliminary economic evaluation of the SAWAL process was made by the Bureau’s Process Evaluation Staff at Avondale, MD. This evaluation was made primarily to determine if sufficient economic potential existed to warrant additional investigations on the process; it was not meant to be an estimate for plant design and construction costs. The estimated fixed capital cost on a first-quarter 1981 basis and for a plant producing 1,000 tons of alumina per day was about $417,000,000. The annual operating cost was about $251 per ton of alumina produced. For comparison, an economic evaluation of the Bayer process showed an operating cost of $250 per ton of alumina. These figures indicate that the SAWAL process has economic potential and that research should be continued.
Summary and Conclusions
Steps for the strong acid-weak acid leaching system were demonstrated and proven to be technically feasible. Implementation of the SAWAL process would eliminate the need for solvent extraction to remove iron and recrystallization steps in the standard HCl-clay leaching process. A flowsheet incorporating common elements from SAWAL and the standard HCl-clay leaching process was proposed. A preliminary economic evaluation was favorable to the SAWAL process.
