Table of Contents
Effective recycling or disposal of flue dusts is a continuing problem, particularly for secondary smelters treating low-grade Cu scrap. The Bureau of Mines investigated a variety of flue dust treatment approaches and demonstrated the technical feasibility of a bench-scale alkaline method that includes leaching, cementation, precipitation, and roasting.
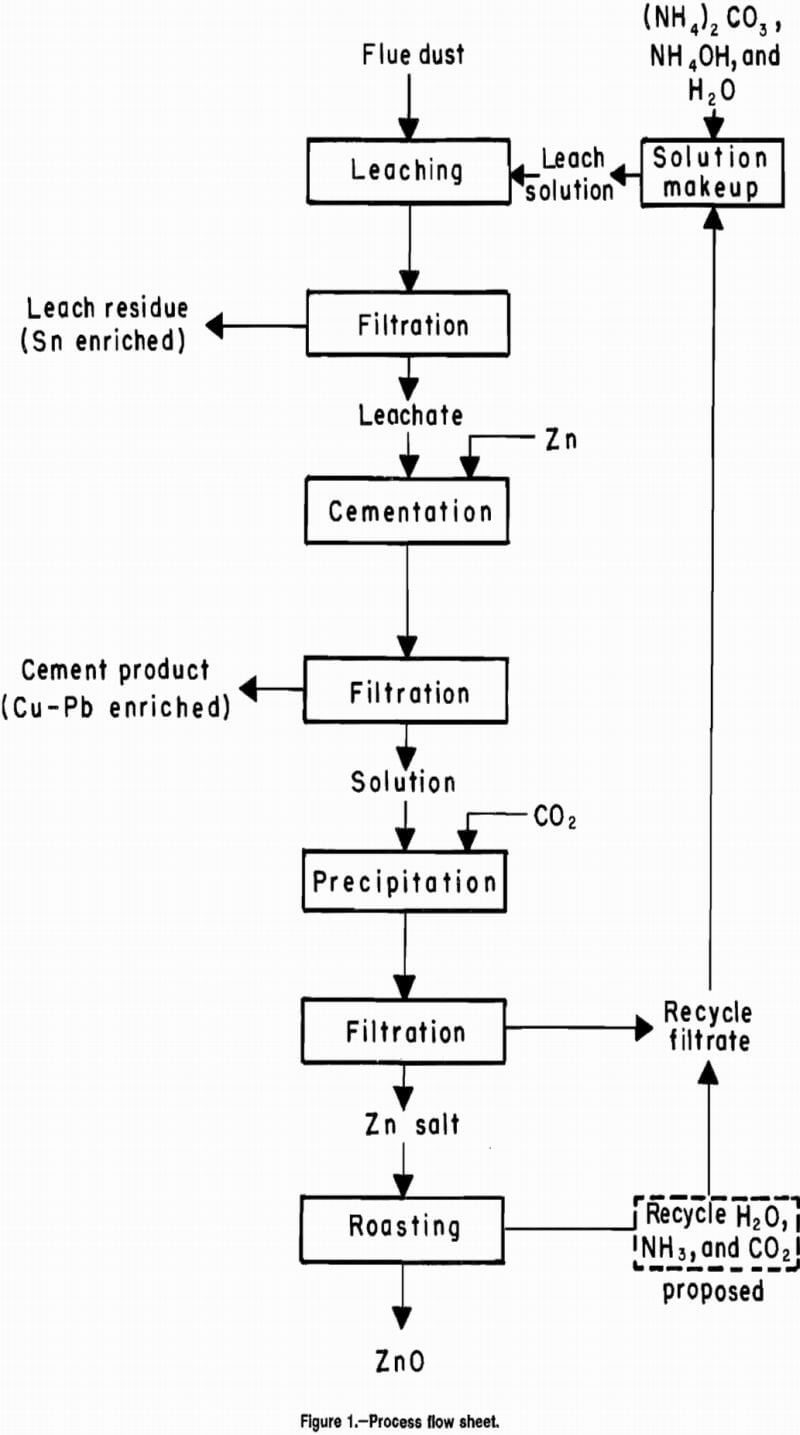
Flue dust was leached at room temperature with an alkaline solution of (NH4)2CO3 and NH4OH to solubilize ZnO and Zn metal. Some copper, lead, tin, and most of the zinc were dissolved in the leach step. Copper, lead, and tin were recovered in the cement product. The Zn in the filtrate was precipitated by pH adjustment to neutral using CO2 injection. The Zn salt was filtered from solution and roasted to give high-purity ZnO.
The leach residue, enriched from 4.3 to 10.4 pct Sn, could find a ready market; the metallic cement product can be recycled back to the copper converter; and the high-purity ZnO product can be sold in several markets, leaving no material for disposal. The possibility for recycling of reagents, lower energy demand, and low environmental impact make this method economically and environmentally attractive for recycling secondary copper smelter flue dusts.
In many manufactured items, copper is combined with other metals and nonmetals and presents a problem in scrap recycling. Pyrometallurgical processing of this scrap, particularly in low-grade forms, generates copious amounts of flue dust and associated collection and disposal problems. In smelting low-grade Cu to black Cu and then converting it to blister Cu, up to 0.3 st of flue dust is generated for each short ton of Cu produced. In the United States, it is estimated that approximately 300,000 st of flue dust is produced annually by secondary copper smelters.
Sales of flue dust for Zn content have been curtailed because of the presence of high concentrations of Pb from solders as well as metal chlorides, probably from Cu wire covered with polyvinyl chloride. A small amount of these Zn-bearing flue dusts meet specifications for use by the agricultural industry as a micronutrient soil additive in commercial fertilizer. Generally, flue dusts containing 8 pct or more Sn can be marketed and processed to recover Sn. Literature review and industry inquiries failed to show wide industry acceptance of any process for treating these dusts. In a study of metallurgical recycling processes, EIC Corp. recommended further study of ZnO recovery from flue dusts.
As part of its program to conserve natural resources, the Bureau initiated a study to explore methods of metal recovery from flue dust. The initial objective was to develop an alkaline process that was environmentally and economically satisfactory. The first approach taken was the investigation of NH4Cl-NH4OH leach solutions. It was determined that an NH4Cl-NH4OH leach solution performed well as a method to solubilize and separate the metal values by cementation, dilution, and precipitation into potentially salable products. It was estimated for this approach that process economics would be acceptable only under ideal circumstances, largely because of the dilution- precipitation procedure used to produce a ZnO product, and the subsequent necessity of reconcentrating the Zn product filtrate to its original volume for recycle. A second problem with this approach was that the ZnO product, contaminated with chloride, would probably only be suitable for use in the electrogalvanizing industry, where chloride is added as an electrolyte component.
The objective of the further research discussed in this report was a short, pioneering effort to study other ammonium salts as alternatives to NH4Cl with little or no emphasis on an indepth study of the chemical kinetics or ammonium ion complex chemistry. The major purpose of this study was to prove technical feasibility of an alternative ammonium salt. The compounds (NH4)2CO3, NH4NO3, and (NH4)2SO4 were all tried as substitutes for NH4Cl. Only (NH4)2CO3 showed promise as an alternative.
The literature also showed that in an (NH4)2CO3 system, Zn solubility is dependent on pH. Base metal carbonates have limited solubility in neutral carbonate solutions at any temperature and precipitated carbonates can be decomposed to oxides readily by heating. Thus, pH adjustment would probably be an effective alternative to the energy intensive dilution-precipitation reconcentration steps necessary with the NH4Cl-NH4OH process. Therefore, a process using ammonium ion and carbonate ion should have a high potential for dissolving both Zn and ZnO in flue dust.
Reagents and Flue Dust
The following reagents were used in studying the leaching of flue dust with an (NH4)2CO3-NH4OH system: reagent-grade (NH4)2CO3, technical-grade NH4OH (27 wt pct NH3), and 30-mesh granular Zn.
A sample of flue dust was obtained from a major secondary copper smelter. Converter flue dust from this smelter is recovered by two collectors in series. The dust from the primary collector causes no disposal problems and was not studied because it has a ready market because of its high Sn content (>8 pct). Flue dust from the secondary collector, a fabric-celled baghouse, was used in this study. This dust is typical of the smelter’s normal operations, has no market value because of its low Sn content (<8 pct), and presents a disposal problem. As a preparatory step to the investigation, a sufficient quantity of this converter flue dust was ground to about 60 mesh (to break up lumps) and blended. The chemical analysis is shown in table 1 and the trace metal content in table 2. The major component identified by X-ray diffraction is zincite with lesser amounts of anglesite and zinc stannate.
Procedure and Development of Operating Parameters
The method developed consisted of first leaching the flue dust with an (NH4)2CO3-NH4OH solution followed by filtering the residue from the pregnant leach liquor. Copper, lead, and trace amounts of tin were removed from the leachate by cementation with granular Zn. The solids were then filtered off and the filtrate was injected with CO2 gas until a neutral pH was reached, which produced a Zn salt precipitate. The Zn salt precipitate product was recovered by filtration and roasted to obtain ZnO. The Zn salt filtrate and the offgases from roasting can be recycled to the leach solution makeup step. In these studies, the beginning and recycle solution makeup was done with (NH4)2CO3, NH4OH, and H2O. A flowsheet was developed for the process (fig. 1).
The leaching, cementation, and precipitation steps were performed in 2-L open reaction kettles with an automatic temperature controlled heating mantle and a propeller stirrer. The filtrations were performed on Whatman No. 541 paper in Buchner funnels using vacuum supplied by a diaphragm pump attached to a suction flask. The roasting was done in porcelain boats in a muffle furnace. Throughout the testing, analytical samples of solids and liquids were taken for assay.
Each step of this approach has one or more parameters that may be varied. To develop this treatment procedure, the following parameters had to be investigated: NH3 and CO2 concentrations, leach temperature, leach time, pulp density, Zn requirement for cementation, cementation temperature, precipitation conditions, and Zn salt roasting time and temperature.
NH3 and CO2 Concentration
Mellor reported that at a concentration of 120 g/L NH3 and 110 g/L CO2, the maximum amount of pure ZnO is solubilized (190 g/L ZnO). If the concentration of CO2 is increased or decreased, the solubility of ZnO will be reduced and dissolved material will precipitate from solution.
The converter flue dust studied contains about 80 g of ZnO in 189 g of dust. The 189 g of solids, when added to 1 L of leach solution, produces a solution set to give maximum Zn removal. For 80 g of pure ZnO, the best concentrations of NH3 and CO2 are 63 and 58 g/L, respectively, based on minimal reagent usage. Since there are other soluble metal oxides in converter flue dust, the necessary NH3 and CO2 concentrations for flue dust will differ from those used with pure ZnO alone.
A series of tests varying NH3 concentrations from 45 to 158 g/L and CO2 concentrations from 19 to 132 g/L were run to determine these different conditions. The leach experiments were all carried out at 85° C for 15 min with stirring [the conditions necessary in the NH4Cl-NH4OH system studied previously. The experimental results indicated that for this flue dust, the highest Zn solubility occurred at 117 g/L NH3 and 94 g/L CO2 concentrations (higher concentrations of NH3 and CO2 did not increase Zn solubility). A solution of 117 g/L NH3 and 94 g/L CO2 was used for all tests described in the following sections.
Leach Temperature and Time
A study of the effect of temperature on the solubility of converter flue dust was conducted using the established NH3 and CO2 concentrations. Over the temperature range of 20° to 85° C, no significant difference was found in the solubility of flue dust. Therefore, room temperature was selected as the best leach temperature because no heat input was required. This temperature was used for all subsequent testing. Leaching experiments were run at 15, 30, and 60 min. Leach times greater than 15 min produced no additional solubilization of metals from flue dust at room temperature.
Pulp Density
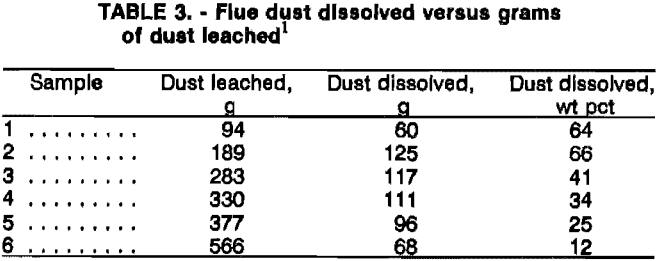
After establishing standard reagent concentrations, leach temperature, and leach time, a series of tests was run using these standard conditions (117 g/L NH3, 94 g/L CO2, room temperature, and a 15-min leach) while varying the pulp density (from 94 to 566 g of flue dust per liter of
leach solution). With up to 189 g of flue dust per liter of leach solution, 64 to 66 pct of the dust was dissolved. At 283 g/L, flue dust solubility had dropped to about 41 pet, and as the pulp density was increased to 566 g/L the solubility continued to decrease (table 3). Therefore, a level of about 189 g of flue dust per liter of leach solution was indicated as being best for maximum leachability under the standard conditions.
Zinc Required for Cementation
Palumbo established that when stoichiometric amounts of Zn were used for cementation, the cement product was very fine grained and tended not to be separated easily during continuous circuit processing tests using an NH4Cl-NH4OH system on this same converter flue dust. Oldright stated that the amount of metal used in cementation is generally in excess of the stoichiometric requirement and the amount in general practice is twice the stoichiometric amount. For this flue dust, 18.9 g of granular Zn per liter (15.8 times the expected stoichiometric requirement) of pregnant leach solution was used. This high level was used because part of the granular Zn will be dissolved by the leach solution in addition to that amount dissolved by the cementation reaction. This amount gave essentially complete Cu, Pb, and Sn removal from the pregnant leach solution and was used in all subsequent testing.
Cementation Temperature
Tests were run at temperatures from ambient to 80° C to determine the best temperature for cementation. At room temperature, cementation is complete in 30 to 45 min, while at 60° C and above, it is complete in approximately 5 min. For reasons of time, a cementation temperature of 60° C appears to be best and was used in further studies.
Precipitation Conditions
Because of the major costs of the NH4Cl-NH4OH process associated with the dilution precipitation step and the subsequent reconcentration of the leach solution prior to recycle, a means of avoiding these steps was sought using an (NH4)2CO3-NH4OH system. Literature indicates that in an ammoniacal carbonate solution, Zn solubility is greatly reduced when the pH of the solution is reduced to neutral (pH 7). Previous studies of NH3 and CO2 concentrations suggested that injecting CO2 lowers the solution pH while at the same time precipitating the Zn as a salt tentatively identified as Zn(NH3)2CO3·ZnO by elemental analysis. Thus, the injection of CO2 gas, with its subsequent pH reduction, was studied as an alternative precipitation procedure.
Results showed that precipitation increased until pH 7 was reached, and then ceased. Approximately 50 pet of the Zn is precipitated by CO2 injection in a single batch operation. Because the Zn remaining in solution is controlled by its solubility at neutral pH, the same amount of Zn will remain in solution in each following cycle throughout the process. After regenerating the leach solution by adding NH3 and CO2, the increased solution solubility will be sufficient to hold the unprecipitated Zn in solution and still have more than enough capacity to dissolve the Zn from the next batch of flue dust. Thus, in succeeding cycles, all the Zn dissolved from each new batch of flue dust will be precipitated when the pH is adjusted to 7.
Roast Time and Temperature
Mellor states that the decomposition of Zn carbonates begins at 90° C, is complete in 1 h at 300° C, and in 30 min at 400° C. The elemental composition of the Zn salt precipitated by CO2 injection fits the stoichiometry for Zn(NH3)2CO3·ZnO indicating that this is probably the species precipitated. While this salt is different from ZnCO3, its decomposition follows a similar temperature profile. Attempts were made to drive off NH3 at low temperatures (100° to 250° C) and then CO2 at 400° C. The test results indicated that at lower temperatures, decomposition was slow, but that both NH3 and CO2 were being evolved. Thus, for time and energy reasons, a 30- min roast at 400° C was chosen as best. The residual product of this roast has been identified by both X-ray diffraction and chemical analysis to be ZnO of greater than 99 pet purity.
Final Operating Parameters
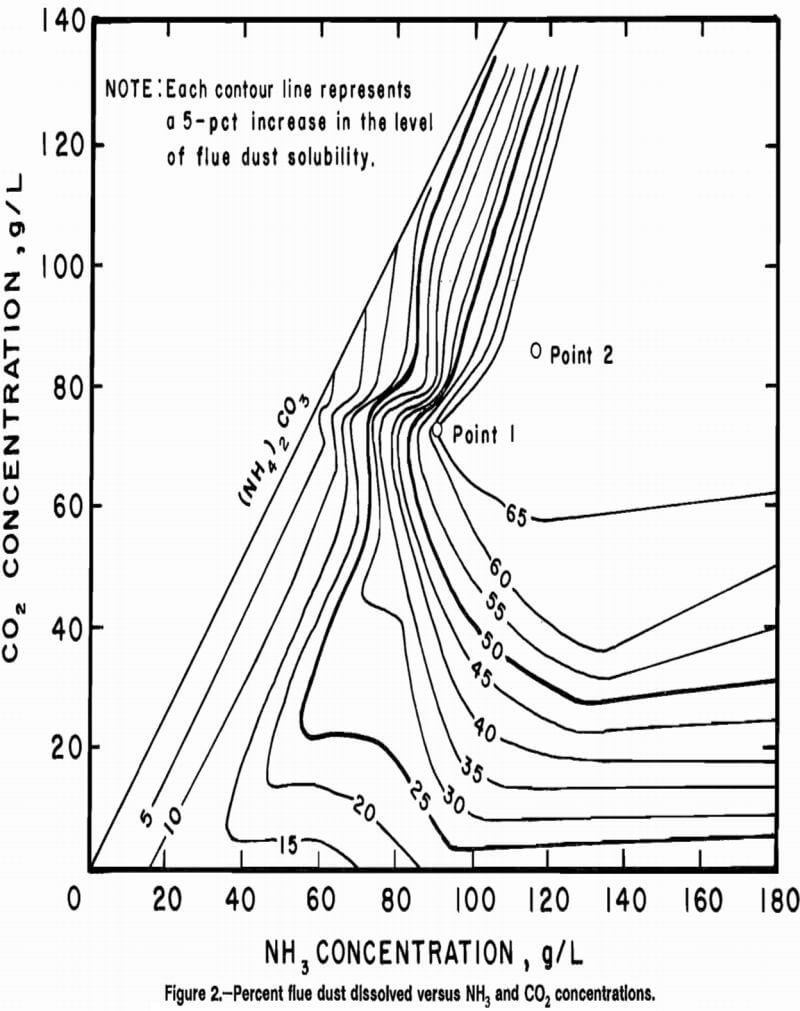
Studies indicated that there was an NH3 and CO2 concentration beyond which the addition of NH3 and/or CO2 did not increase significantly the solubility of flue dust. However, the addition of NH3 and CO2 beyond this point significantly affected the solubility of Cu. Therefore, a second set of tests was run to determine the effect of NH3 and CO2 on Cu and Zn solubility. In this series of about 50 leach tests, the NH3 concentration was varied from 18 to 189 g/L and CO2 concentration from 0 to 132 g/L, using (NH4)2CO3 and NH4OH. The data derived from this study are shown in Figure 2. This contour plot shows the percent of converter flue dust solubilized versus the initial NH3 and CO2 concentrations. Each contour line represents a 5-pct-increment level of dust solubility. Beyond the 65-pct contour, additional CO2 or NH3 caused no increase in solubility of the flue dust.
To understand this figure, it is best to examine solubility of flue dust while holding one concentration constant and varying the other. For example, if the C02 concentration is held constant at 85 g/L, it is observed that as NH3 increases from its limiting concentration in (NH4)2CO3 (-65 g/L), the solubility of the flue dust increases to 65 pct at 106 g/L NH3. Beyond this point, the solubility is constant (within experimental accuracy) in the range of 65 to 67 pct. Holding the NH3 concentration constant at 117 g/L, as CO2 increases from 0 to 58 g/L, it is seen that flue dust solubility increases from about 20 pet to 65 pct. Further increases in CO2 from 58 to 110 g/L cause no further increase in flue dust solubility. As the CO2 increases from 110 to 132 g/L, the solubility of flue dust decreases from 65 to 45 pct.
The contour plot also shows that the maximum solubility of flue dust (mainly Cu and Zn) will be reached under these experimental conditions at minimum levels of NH3 and CO2 of 91 and 72 g/L, respectively (point 1). Because the maximum solubility of Cu is also desired and because of the extreme inhomogeneity of the flue dust, some margin of excess reagent was required. Analytical data indicated that concentrations of 117 g/L NH3 and 85 g/L CO2 (point 2, figure 2) will be acceptable for maximum Cu and Zn solubilization, and give some excess margin of reagent required by the flue dust inhomogeneity.
All other parameters were used as previously determined. Thus, the operating parameters for testing the feasibility of the process are a 1-L leach solution containing 117 g/L NH3 and 85 g/L CO2 to leach 189 g of flue dust at room temperature for 15 min; 18.9 g of 30- mesh granular Zn at 60° C and reacted with the pregnant leach solution for 5 min for cementation of Cu, Pb, and Sn; precipitating the Zn salt from the cementation leachate by adjusting the pH to neutral (pH 7) using CO2 gas; and roasting the Zn salt for 30 min at 400° C to give a >99- pct-pure ZnO product. These operating conditions are adequate to accommodate the inhomogeneity of the converter flue dust.
Final Tests of Leaching Procedure
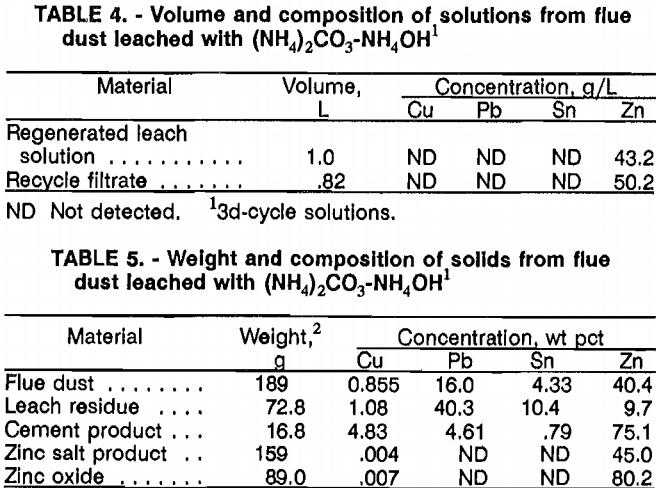
Using the parameters described and the flowsheet shown in figure 1, a series consisting of an initial leach test plus two complete locked cycles was run in a semibatch mode to determine the effectiveness of each step, a material balance, and the feasibility of recycling the Zn salt filtrate back to the beginning of the next leach cycle. After the first cycle, the Zn product filtrate was analyzed for NH3 and CO2 concentration. The solution was then adjusted back to the starting concentration using (NH4)2CO3, NH4OH, and H2O. This solution was used to leach a second 189-g batch of flue dust. Again, after completing Zn salt precipitation, the filtrate solution was analyzed for NH3 and CO2 and adjusted to its original concentration. A third 189-g batch of converter flue dust was then leached, and after the Zn salt was separated, the process was stopped. Samples were taken from each solution and solid for analysis.
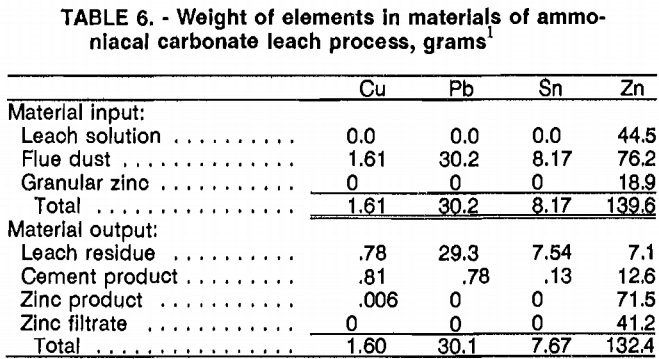
The second and third pregnant leachates had the same concentrations of Cu, Pb, Sn, and Zn, demonstrating that the system had approached equilibrium, that all steps worked effectively, and that the test would provide reliable data for a material balance and a demonstration of the feasibility of recycling the leach solution. The analysis of the third-cycle liquids and solids (tables 4 and 5) show that 51 pct of the Cu, 3 pct of the Pb, 7.7 pct of the Sn, and 91 pct of the Zn are dissolved in the leach step. Of these amounts, 98 pct of the leached Cu, 100 pct of the leached Pb, and 21 pct of the leached Sn are recovered in the cement product. The Zn salt product contains 94 pct of the Zn dissolved from the flue dust. There is no evidence of Zn being lost during roasting of the salt.
The high Zn content of the cement product indicates that too much Zn was used for cementation. It is estimated from the data that about 1.2 g of Zn would have
been the stoichiometric amount to cement out the Cu, Pb, and Sn in the leach solution. Since prudent practice uses an amount in excess of the stoichiometric amount, usually double, the procedure should have needed 2.4 g Zn. However, because (NH4)2CO3-NH4OH solutions dissolve metallic Zn, a greater amount would have been needed. About 6.3 g dissolved during cementation; 1.2 g dissolved by the cementation reaction, and the remaining 5.1 g by the leach solution. It appears that a value of about 8 g of granular Zn per liter of pregnant leach solution would have been more appropriate.
Except for this excess of Zn for cementation, the process performed as expected. The leach residue, now enriched from 4.3 to 10.4 pct Sn, would be a readily salable product. The metallic cement product containing 50 pct of the Cu can be recycled back to the copper converter and the ZnO product from roasting can be sold as an electro- galvanizing feedstock, leaving no material for disposal.
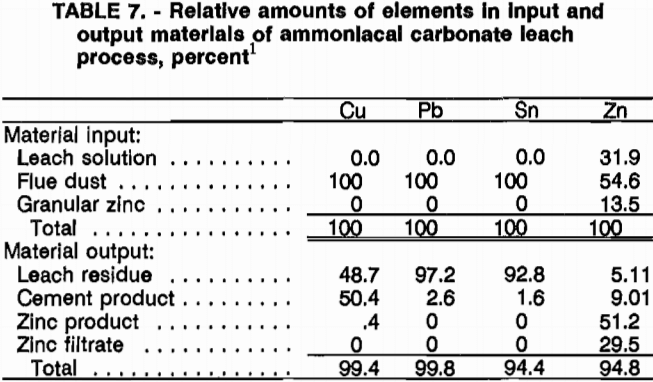
The material balance for the procedure is taken from the third cycle data (tables 6 and 7). The data show that the Cu, Pb, and Sn come from the flue dust, while 32 pct of the Zn comes from the recycled leach solution (the amount soluble at pH 7), 55 pct from the flue dust, and about 14 pct from the cementation Zn. In the output streams, Cu is about evenly split between cement product (50 pct) and leach residue (49 pct), with a trace in the ZnO product (0.4 pct). A little Pb was dissolved with about 3 pet reporting in the cement product and the remaining 97 pct in the leach residue. Most of the Sn remains in the residue (93 pct) with about 2 pct in the cement product and about 5 pct unaccounted for. Zinc appears in all outputs, 5 pct remaining in the leach residue (probably as zinc ferrite), 9 pct in the cement product (unreacted granular Zn), 51 pct in the Zn product, and about 30 pet in the final filtrate, which will be recycled back to leach solution makeup. The material balance is good with 94.4 to 99.8 pct of the input elements being accounted for in the outputs.
The recyclability of the leach solution was successfully demonstrated. On the first leach cycle, only 65 pct of the Zn from the flue dust appeared in the Zn product. Its level of recovery is limited by the solubility of Zn in ammoniacal carbonate solution at pH 7. By the third cycle, the system is now approaching equilibrium with 94 pct of the Zn from each batch of flue dust reporting to the ZnO product. In a continuous circuit process, there will always be a certain amount of Zn carried in the recycle solution because of its solubility. However, as the same amount of Zn is dissolved from the flue dust and the cementation Zn, the resultant ZnO product will continue to contain 94 pct of the Zn dissolved from the flue dust. In a pilot or larger plant, this amount will approach 100 pct. The difference is due to small batch processing solution losses.
Important advantages of the procedure are that it con¬serves energy and that the ZnO product is not contaminated with chlorine. The energy cost of keeping the solutions at 80° C and particularly the step of reconcentration of the leach solution by reverse osmosis and/or thermal evaporation after the dilution-precipitation in the NH4Cl-NH4OH process are avoided. The ammonium carbonate process, even with the energy requirement for roasting, is likely to perform significantly better economically than the previously tested NH4Cl-NH4OH process.
Summary and Conclusions
An alkaline method to recover ZnO and metal values from secondary copper smelter flue dusts was developed. The method consists of an alkaline (NH4)2CO3-NH4OH leach of the flue dust; recovery of soluble Cu, Pb, and Sn by cementation with Zn metal; and recovery of ZnO by adjusting the pH of the filtrate with CO2 to precipitate a Zn salt followed by filtration and roasting. Leaching of a dust containing 4.3 pct Sn upgraded it to a residue containing 10.4 pct Sn, which is a salable byproduct. The cement product can be recycled back to the copper converter, as it is primarily metallic. The ZnO product has a small market in the agricultural sector as a micro-nutrient soil additive and an animal feed additive. The best potential market seems to be as a feed stock for electrogalvanizing.
The system is technically sound and feasible as all solid products and byproducts are either saleable or can be recycled back to the smelter furnace and all liquid streams can be recycled. There are fewer hazardous materials to impact the environment. Finally, the energy demand is probably much less than that of the NH4Cl-NH4OH system. This research illustrates that waste materials such as secondary copper smelter flue dusts, which pose significant disposal or environmental problems, can be processed to recover valuable metal resources for sale or recycle with little or no material left for disposal.
