Table of Contents
As part of its mission to maintain an adequate supply of minerals to meet national needs and maximize mineral production from domestic sources, the Bureau of Mines is investigating the recovery of alumina by hydrochloric acid leaching of kaolinitic clay. Most iron impurities are leached from the clay along with the alumina and must be removed before or during crystallization to aluminum chloride hexahydrate. The thermal decomposition step then provides an alumina product of acceptable iron analysis. Miniplant studies of a Bureau developed solvent extraction technique, using a tertiary amine in kerosene diluent, have demonstrated that the iron content in aluminum chloride leach liquors can be reduced to levels of less than 0.007 wt-pct (7 ppm), expressed as Fe2O3. Entrained and dissolved organic matter in the purified pregnant leach liquor from the solvent extraction circuit can be reduced to less than 2 ppm by using a fabric-packed coalescer and subsequent activated carbon treatment. Investigators discovered that a steady buildup of zinc accumulated in the solvent phase within the extraction circuit, proportional to the amount of leach liquor treated. The solvent phase zinc analysis increased by 0.01 wt-pct ZnO for each 1,000 gal of pregnant leach liquor purified.
Solvent extraction (SX) removal of iron from about 26,000 gal of liquor from a hydrochloric acid leach of calcined kaolinitic clay was carried out on a miniplant scale at the Bureau’s Boulder City (Nev.) Engineering Laboratory during two major test programs. The first test program was run under four different sets of operating conditions during 8 days of operation in April and May 1977. These four tests were characterized by a detailed sampling and process evaluation study. The second test program, run during 7 days in April 1978, was conducted entirely under one set of operating conditions. The primary purpose of this second run was to provide additional purified pregnant leach liquor for subsequent crystallization tests and to verify the results of the first run. Sampling was minimal.
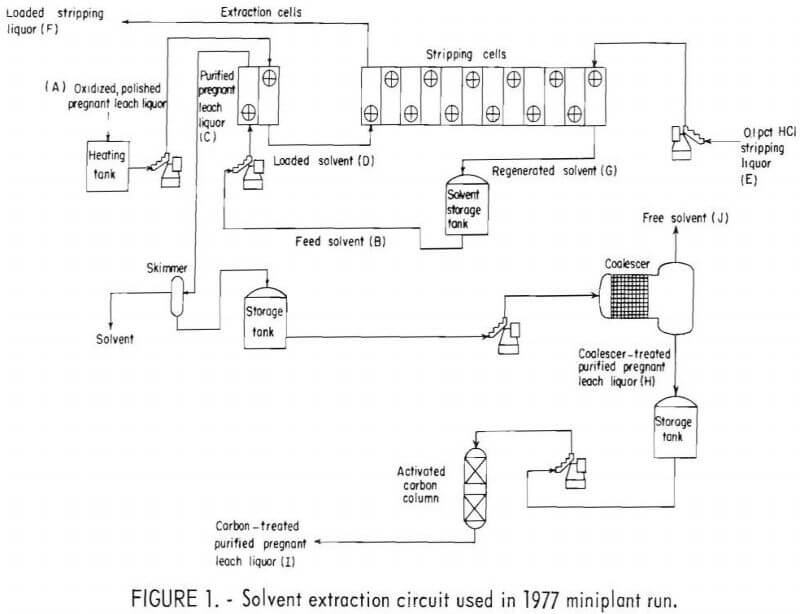
The two SX runs included a closely controlled operation of the extraction and stripping circuits. Bench-scale testing at the Bureau’s Reno (Nev.) Research Center dictated the design of the SX circuit in the miniplant. The circuit, shown in figure 1, was used for two of the 1977 tests. Each flow-stream on the figure is labeled by title and code letter.
Because ferrous iron is not removed by the extraction circuit, it was necessary to oxidize essentially all the ferrous iron (61 ppm) to the ferric state. This was accomplished by direct chlorination to oxidize the ferrous iron to a Fe+++:Fe++ ratio of >5,000:1. The resulting ferrous iron content after oxidation was 0.3 ppm, expressed as Fe2O3.
The objectives of the 1977 solvent extraction study were to redesign and operate the clay-hydrochloric acid miniplant SX circuit to produce a purified pregnant leach liquor (I) suitable for crystallization, and to develop optimum operating parameters for the process. Specific steps and goals follow:
- To set phase ratios and the number of mixer-settlers in accordance with laboratory equilibrium studies to attain maximum efficiency in production of a purified pregnant leach liquor containing less than 0.0012 wt-pct total Fe and Fe2O3. On a “bone-dry” basis, this represents a Fe2O3 content
of 0.015 pct, which was set as the maximum limit for iron in the final alumina product from the miniplant. This level was appropriate at the time, but ignored the effect of crystallization on the iron content of the aluminum chloride hexahydrate crystals. An additional step was the production of stripping circuit waste liquor, loaded stripping liquor (F), containing more than 4.2 pct total Fe as Fe2O3—the recommended operating level from laboratory equilibrium study. - To operate and sample the circuit for developing material balance models at the various test conditions, and to determine dynamic equilibrium distribution of iron between aqueous and organic phases. Comparison of these distribution data with those of the bench study serves to indicate both the reliability of the data and to demonstrate how the system can be scaled up.
- To determine the effect of regenerating and recycling the organic phase. Studies on the use of rubber-lined apparatus in processing of purified pregnant liquor through the crystallization stage indicated that even a low level of organic matter present in the purified pregnant leach liquor would cause severe deterioration of the linings. Organic contamination of purified pregnant leach liquors that resulted in earlier miniplant tests was believed to be far in excess of the estimated acceptable limits of <5 ppm because those liquors were not treated by any method of organics removal. Recent technology for minimizing emulsion formation and improving phase separation in SX mixer-settlers suggested three likely avenues which were incorporated in the circuit design: modification of standard mixer impeller turbines, by removing the upper blades; operation of turbines at speeds below a formulated maximum value that is proportional to impeller diameter; and installation of two slot-ted dams (“picket fences”) in the settler section of each cell, just behind the mixer system.
It was also necessary to add equipment to remove entrained organic matter from the purified pregnant leach liquor (C). For this purpose, the liquor was first passed through a skimmer tank, then to a cloth-packed coalescer and, finally, treated with activated carbon. All 26,000 gal of purified leach liquor were either passed through packed carbon columns or mixed with carbon and filtered. Sampling was carried out to determine organics removal efficiency of these steps. Carbon from the packed carbon columns was analyzed to relate organics loading of carbon to location within the packed columns.
The objective of the 1978 run was to purify additional leach liquor to provide feed for an extensive hydrochloric acid gas-sparging crystallization test program in the Bureau miniplant. The circuitry and operational procedures were substantially those developed in the 1977 run.
Equipment and Procedures
The miniplant solvent extraction operations in 1977 and 1978 utilized circuitry developed from laboratory testing. An overall design, as shown in figure 1, was used under test conditions listed in table 1.
Solvent Extraction Mixer-Settlers
The mixer-settler arrangement provided 2 cells for extraction purposes, and 12 cells for stripping (fig. 1). This circuit was used in 1977 tests 3 and 4, with an extraction ratio of 5:1 aqueous to solvent phase and a stripping ratio of 4.25:1 solvent phase to aqueous phase. It was the most efficient circuit tested. As shown in table 1, two other test configurations were used: 3 extraction cells with 9 stripping cells (1977 tests 1 and 2), and two parallel 3-cell extraction units, with a single 12-cell stripping unit (1978 run). Tests 1 and 4 (1977 run) were run at feed rates of 25.5 gph of polished pregnant leach liquor (A), while the remaining 1977 and 1978 tests were run at 51 gph. The higher feed rate of 51 gph corresponds to a design ratio of 0.9 gpm/sq ft of settler area. The flow rate recommended by the manufacturer of the organic extractant was approximately 1 gpm/sq ft of settler area.
Solvent extraction cells were Denver Laboratory Mixer-Settler Units, with approximately 2 gal-mixer volume, 1-sq-ft settler area, and 0.8 cu-ft settler volume. Each mixer is 7 by 7 inches, and each settler is 7 by 22 inches. Both mixers and settlers have 10-inch operating depths. Each unit had a bypass arrangement which could be used to return a portion of either the settler aqueous discharge or the solvent discharge back to the mixer. This arrangement can be used to increase the proportion of one phase in the mixer at the expense of the other. In this way, even with an extraction ratio of 5 parts aqueous to 1 part solvent, it was possible to produce a “solvent continuum” liquor in the first extraction cell mixer. The identity of the continuous phase was determined by using a simple conductivity meter. Low conductivity showed that the solvent phase was the continuum, high conductivity indicated the reverse. This was accomplished by starting the unit, with only solvent going to the mixer, and recycling a large part of the settler organic discharge. When a definite “solvent continuum” existed, the aqueous feed was introduced to the mixer. A condition of “solvent continuum” in the mixer provided visibly clearer solvent and aqueous phases in the settler. Thus, better phase separation was expected. However, recirculating the solvent phase required higher impeller speed which might have, in turn, actually increased solvent entrainment in the aqueous phase. The net effect of using “solvent continuum” mixing in the extraction circuit was not established.
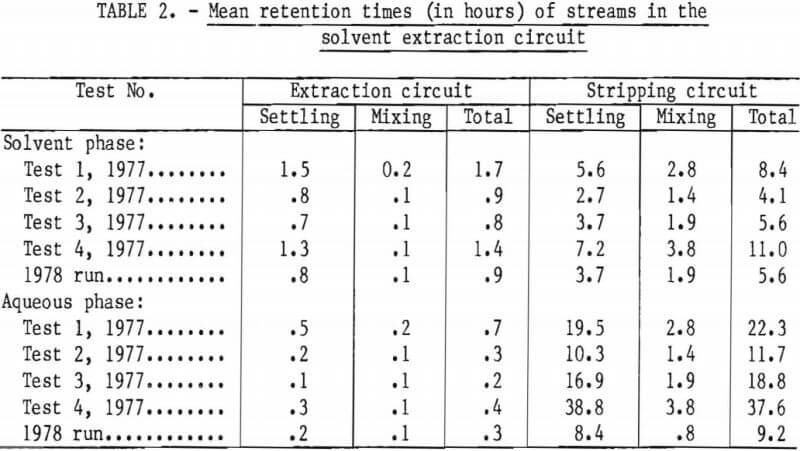
The height in the settler of the contact plane between the solvent and the aqueous phase was set for each settler unit to minimize entrainment of one phase in the other in the final products from the solvent extraction circuit. The settler unit of the last cell in each circuit process stream had a thick layer of that particular process stream liquor. Thus, the cell discharging the purified pregnant leach liquor (C) had an aqueous layer about 8 inches thick below a thin solvent layer, but the cell discharging loaded solvent (D) had a solvent layer about 8 inches thick above a thin aqueous layer. This rationale was also applied to the stripping cells. For intermediate cells in the circuit, the relative thicknesses of the aqueous and solvent layers were set proportional to the cells location in the circuit. For example, the middle cell would have aqueous and solvent layers of equal thickness. Mean retention times for each circuit stream, that is, the time for a single displacement of a flowstream through a circuit, are given in table 2. These data are provided for use in possible scaleup of the solvent extraction circuit to commercial size.
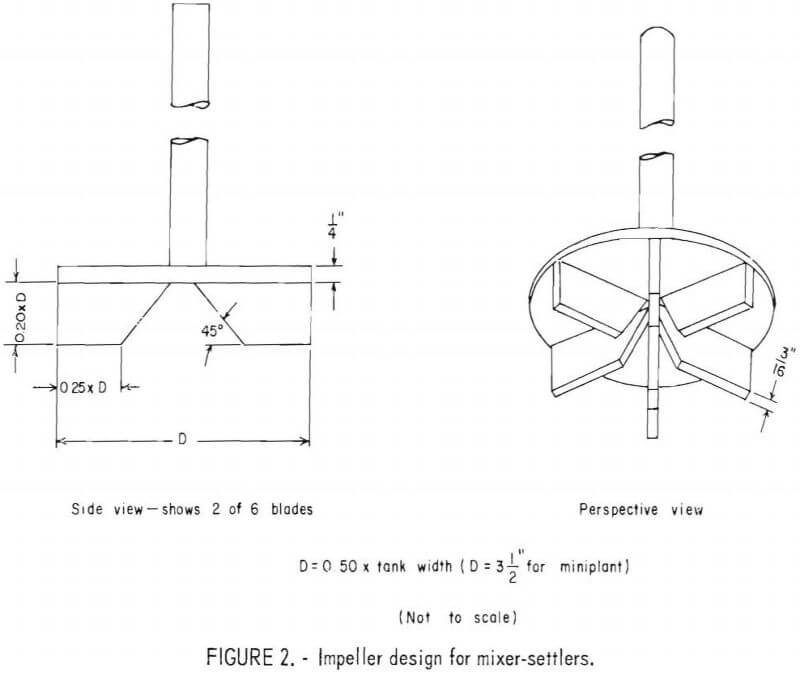
In an attempt to decrease entrainment of solvent in the aqueous phase, the SX impellers in the original equipment were modified by removing the blades above the turbine plate (disk); and increasing the overall depth and diameter of the underside blades. This modified impeller design is shown in figure 2. According to T. Plouf, Chief metallurgist at the Denver Equipment Co., the design was made to conform to new recommendations by the SX cell manufacturer and probably reflects design data developed by Agers and Dement. However, after this solvent extraction study was completed, it was learned that C. Hanson, in working on design improvements for a Zambian copper plant, had found that impeller size has very little influence on stage efficiency, but does substantially influence entrainment, and that the optimum impeller diameter should be only one-third the tank diameter.
Mixer-settler speeds were set just high enough to maintain steady pumping from one cell to the next and to provide mixing of the two phases, while avoiding such violent agitation that the phases would homogenize and fail to disengage in the settler units. Impeller tip speeds were between 6 and 9 fps.
To improve disengagement of phases, two “picket fences” (dams with vertical slots) were installed just behind the mixer section of each cell. The slots in the dams were intended to induce coalescence by channeling the flow pattern to produce an elongated venturi effect, thereby causing suspended droplets to combine. 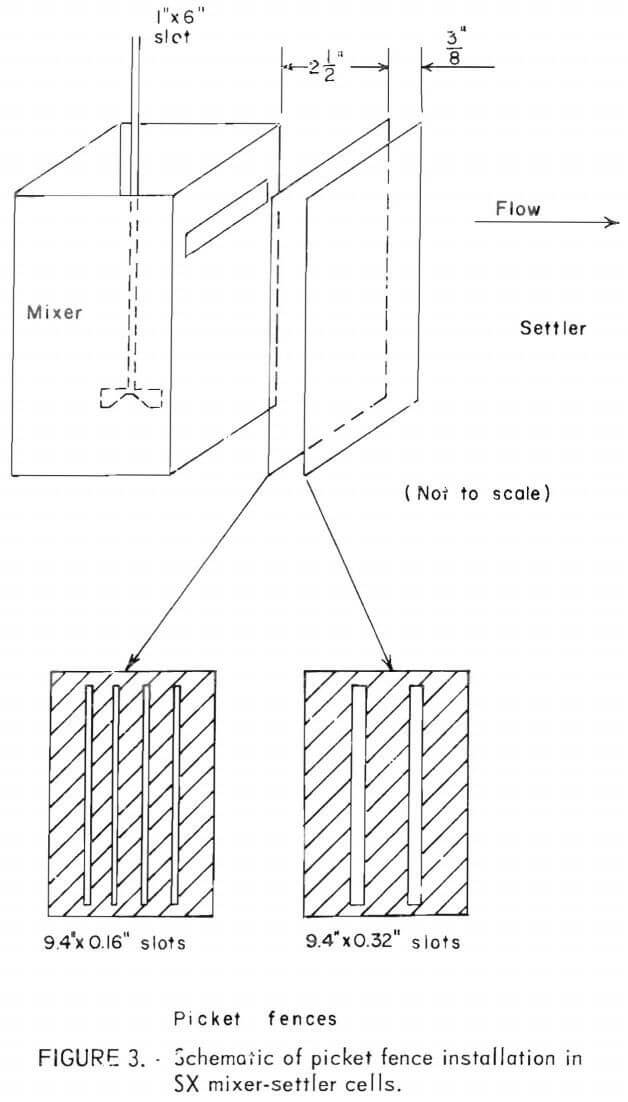 The two slotted dams shown in figure 3 were dimensioned so that the mean flow rate through them was equal to the flow rate through the 1- by 6-inch slot between the mixer and settler portions of the cell. This results in the first dam having four slots of about 0.16-inch width, and the second dam having two slots of about 0.32-inch width. The first dam was 2½ inches downstream from the mixer, the second dam was 3/8-inch further downstream.
The two slotted dams shown in figure 3 were dimensioned so that the mean flow rate through them was equal to the flow rate through the 1- by 6-inch slot between the mixer and settler portions of the cell. This results in the first dam having four slots of about 0.16-inch width, and the second dam having two slots of about 0.32-inch width. The first dam was 2½ inches downstream from the mixer, the second dam was 3/8-inch further downstream.
Polished pregnant leach liquor (A) at 40° C was fed to the extraction circuit. Temperature of the liquor was held near 40° C by glass-jacketed immersion heaters, located in the settler sections. This ambient temperature was chosen to correspond to the expected temperature of the polished pregnant leach liquor feed (A) in a full scale plant. An elevated temperature has the advantage of improving phase separation in the settler units. No heaters were used in the stripping cells. The temperature decreased from about 35° C in the first stripping cell to about 25° C in the last cell.
The solvent consisted of 15 vol-pct Alamine 336 manufactured by the Henkle Corp., Tucson, Ariz., 10 vol-pct decanol EXX1361 Decyl Alcohol Formula 9120, manufactured by Exxon Chemical Co., (commercial-grade mixed isomers of decyl alcohol) and 75 vol-pct Chevron Pearl Kerosene Product No. 217105, manufactured by Standard Oil of California. The Alamine 336 was chosen because of its loading capacity and selectivity characteristics; decanol functions as a modifier to suppress formation of a third phase under conditions of full loading of the organic phase.
Coalescer
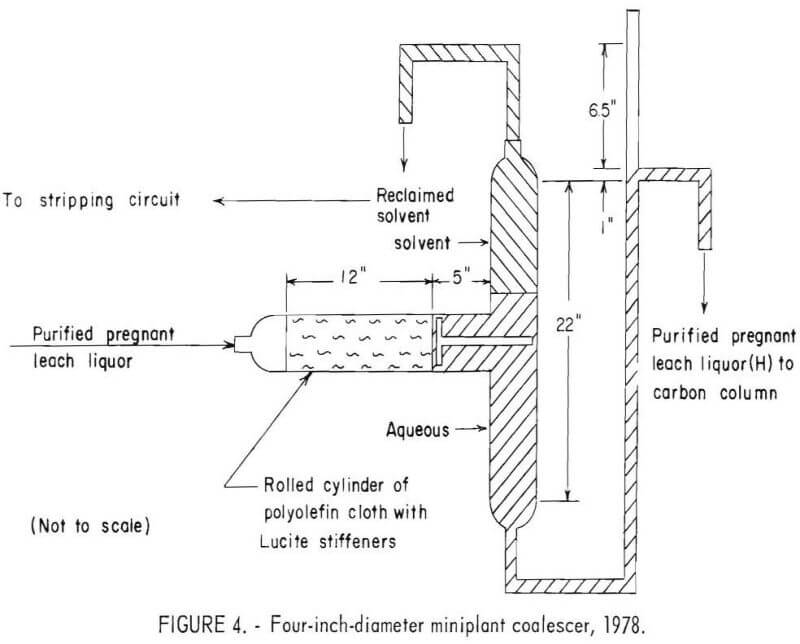
The coalescer consisted of a cloth-packed, horizontal glass cylinder, with a 4-inch ID, through which purified pregnant leach liquor (C) was passed. As shown in figure 4, the horizontal cylinder was connected to a vertical cylinder to form a T shape in which the coalesced solvent separated from the purified pregnant leach liquor. A 1-foot-wide polyolefin multifilament cloth was rolled with ¼-inch by ¼-inch by 1-foot Lucite stiffeners to form the cloth packing.
Carbon Column
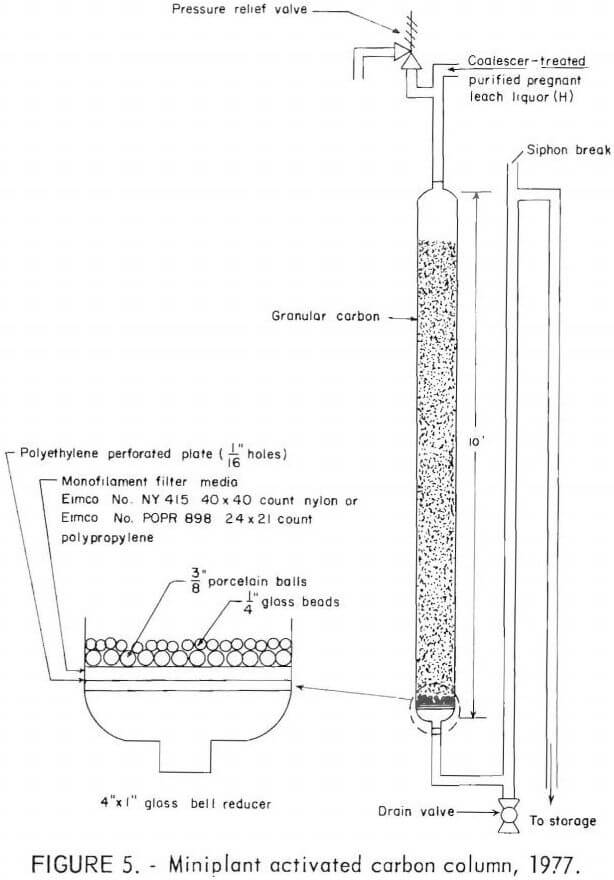
During the 1977 tests, two 10-foot-long glass columns, with 4-inch ID’s, packed with granular activated carbon were used alternately to remove organics from the coalescer-treated purified pregnant leach liquor (H), as shown in figure 5. Granular minus 12- plus 30-mesh activated coconut-hull charcoal (Westates carbon) was used in three tests. Each column was charged with 16.5 pounds of carbon. Air introduced while charging the granular carbon dry remained as air bubbles within the carbon section and caused liquor channeling and high-column back-pressure during operation. Soaking the granular carbon in water before loading it into the column kept the carbon section nearly free of air bubbles. Operating parameters are given in table 3.
In the 1978 run, two 10-foot-long by 6-inch-ID columns were run in parallel at a rate of 51 gph each.
The carbon columns were backwashed with water to remove any fine carbon before operation.
Nevertheless, the carbon-treated purified pregnant leach liquor (I) from the column was filtered as a precaution. It was filtered with a plate-and-frame filter in the 1977 run, whereas in the 1978 run, it was filtered with a 10 m cartridge filter.
Carbon Mixing-Filtration
An alternative method of organic removal entailed mixing powdered activated redwood carbon (PACarb WW-P manufactured by Pacific Carbons, Inc.) with the coalescer-treated purified pregnant leach liquor (H) in a 2,500-gal tank, then removing the carbon by filtration. To insure attainment of equilibrium conditions for organic removal, a minimum 4-hour mixing period was used in each test.
For filtration, two plate-and-frame filters, with areas of 7.7 and 12.9 square feet, were used. A filter cloth of monofilament polypropylene was used and supplemented by an asbestos precoat of about 0.05 pound asbestos per square foot of filtration area. Four carbon filtration tests were run, each using approximately 0.1 pound of carbon per gallon of coalescer-treated purified pregnant leach liquor (H).
 Sampling
Sampling
Extraction and Stripping Samples
Samples were taken from the extraction and stripping sequence designed to cause minimum disturbance to the system. Iron analysis of each of the solvent and aqueous feeds and discharges were monitored every 6 to 8 hours, except for the 0.1-pct-HCl stripping liquor (E) which did not change. In addition, two complete series of individual cell samples were taken near the end of each of the four tests for dynamic equilibrium data. In 1978, however, sampling was considerably simplified. A sample of the entire polished pregnant leach liquor (A) product was taken before the run started, and samples of the carbon-purified pregnant leach liquor (I) and loaded stripping liquor (F) were taken from the entire products after the run. Samples of loaded solvent (D) and regenerated solvent (G) were taken near the end of the run.
Coalescer Discharge Samples
The discharge of coalescer-treated purified pregnant leach liquor (H) from the coalescer was sampled in 300-ml amounts near the end of each test series. Samples were taken directly in 1,000-ml flasks which were then fused shut. Organics content was determined by analyzing the entire sample, including the interior wetted surface of the flask.
Organics were extracted with CCl4 from the air-dried carbon samples in a Soxhlet extraction apparatus and from the liquor samples by washing three times in a separating funnel. Analyses were made by infrared spectrophotometry, using the carbon-hydrogen bond with modifications developed by the Bureau. Standards were developed for each of the components of the solvent singly, as well as for their actual mixture composition.
Carbon Column Samples
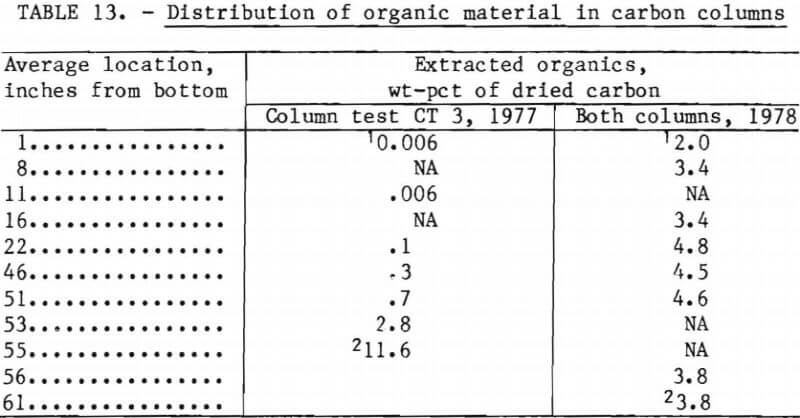
The carbon-treated purified pregnant leach liquor (I) discharge from the carbon column was sampled in the same way as the coalescer discharge, but only feed (H) and discharge (I) samples from carbon column test CT 3 were analyzed in 1977. After a predetermined amount of liquor had been fed to the column, it was drained of liquor and flushed. The flushing rate was between 1 and 3 liters per minute, using 300 gal of water adjusted to a pH of 3.0 with hydrochloric acid. This wash was then drained out of the column, and the carbon bed was carefully separated into 2-inch fractions. These fractions were analyzed for contained organic matter (test CT 3 column in 1977 and both columns in 1978).
Carbon Mixing-Filtration Samples
Some coalescer-treated pregnant leach liquor (H) was not fed to the carbon columns, but was stored in tanks for carbon mixing-filtration treatment. Contents of these storage tanks were vigorously stirred for several hours to insure dispersion of organics; samples were taken, and then the powdered carbon was added. After a mixing step, followed by removal of the powdered carbon filtration, samples of the filtrate were taken. Four batches were thus processed and sampled.
Experimental Results
Iron Extraction
The total iron content of the pregnant leach liquor purified during the 1977 run averaged 0.0005 wt-pct Fe2O3. On a “bone-dry” basis, the solids resulting from the desication of the purified pregnant leach liquor would contain 0.006 wt-pct Fe2O3, which is well below the target value of 0.015 pct for maximum iron content in the final alumina product. A pregnant leach liquor (A) purified to <0.0012 wt-pct Fe2O3, if evaporated and decomposed in its entirety, will yield a product containing <0.015 pct Fe2O3. This level seemed appropriate at the time of these tests; however, depending on the amount of iron rejected in crystallization, significantly higher iron content could be tolerated in purified liquors.
Table 4 presents results of analyses for iron and alumina in the purified liquors produced under the test conditions listed in table 1. These data show that two cells (1977 tests 3 and 4) were as effective in extracting iron as three cells (1977 tests 1 and 2), and raising pregnant liquor throughput from 25.5 gph (1977 test 1 and 4) to 51 gph (1977 test 2 and 3) did not lower efficiency of the iron extraction.
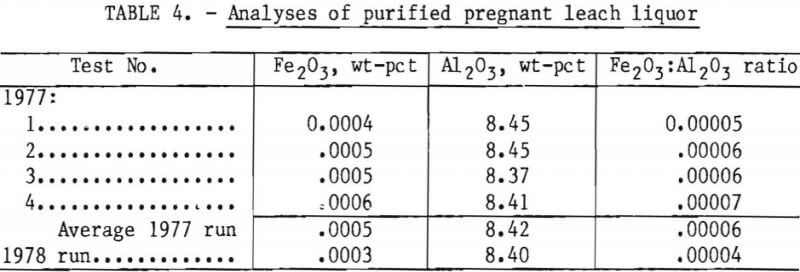
Iron analyses of the contents of individual extraction cells in tests 1 and 4 were used to determine the position of dynamic equilibrium for distribution of iron between solvent and aqueous phases. Iron distribution between solvent and aqueous phases are compared between a two-stage (test 4) and a three-stage (test 1) extraction unit in table 5 and figure 6. In addition, the figure shows the close agreement between the iron distribution points attained under miniplant conditions and the iron equilibrium curve reported in the Bureau studies.
Iron Stripping
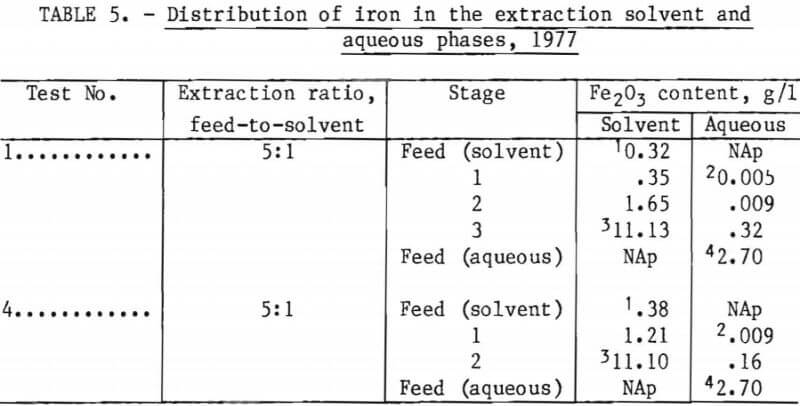
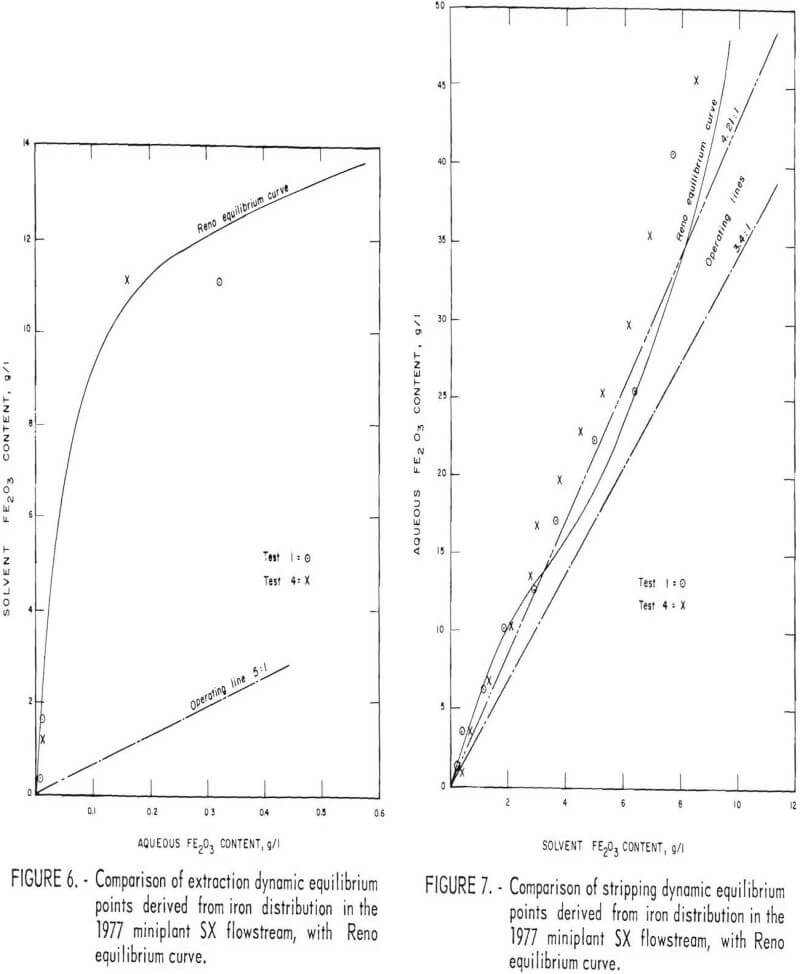
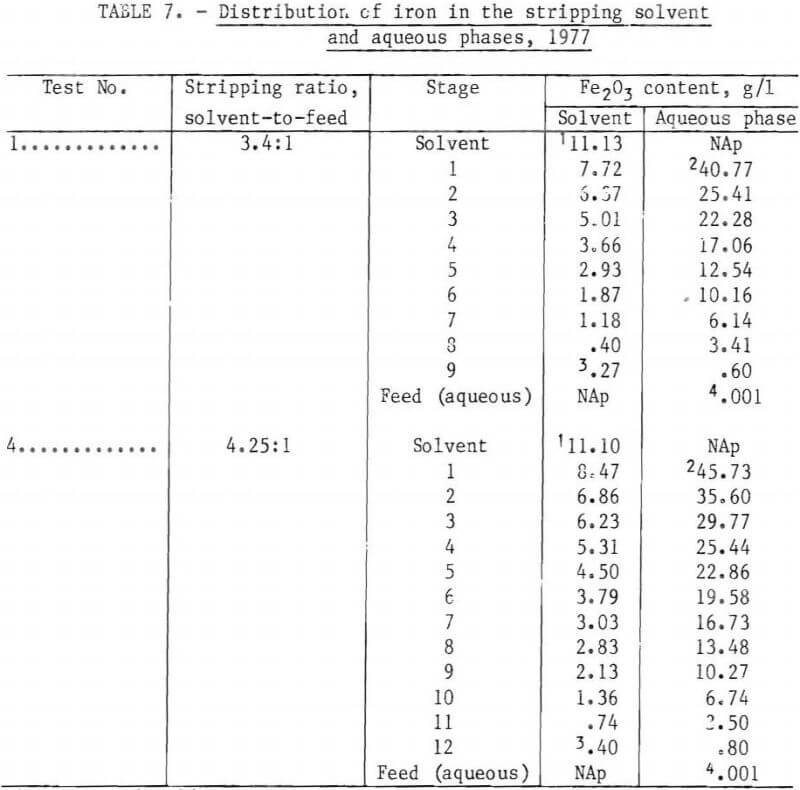
Production of a stripping circuit waste liquor containing 42 g/l Fe2O3 was achieved at a stripping ratio of 3.4:1 loaded solvent to fresh aqueous (0.1-wt-pct-HCl solution), as predicted by laboratory studies. As shown in table 6, this concentration was increased between 48 and 52 g/l (between 4.5 and 4.9 wt-pct Fe2O3) in tests 3 and 4, with a stripping ratio of 4.25:1.
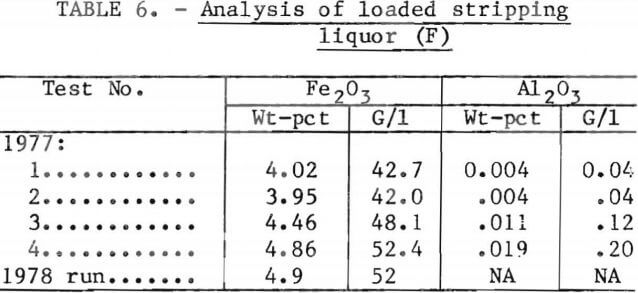
Steady-state distribution of iron between the solvent and aqueous phases in the stripping section is shown in table 7 and figure 7 for tests 1 and 4. Comparison is made of a 9-stage, 3.4:1 stripping ratio circuit to a 12-stage, 4.25:1 stripping ratio. Close agreement is shown between the laboratory equilibrium curve and the miniplant iron distribution points. The close proximity of the 4.25 stripping ratio operating line to the equilibrium curve suggests that the stripping liquor iron content of 4.9 pct Fe2O3 obtained in test 4 is a maximum value, at least for a 0.1-wt-pct-HCl strip solution.
A proposed disposition of this loaded stripping liquor (F) is to react it in a separate circuit with calcined kaolinitic clay. This will extract alumina and at the same time transfer the iron to the residue fraction.
Material Balance
Material balances for the extraction and stripping operations are shown on figures 8 and 9 for tests 1 and 4, respectively. These balances were constructed from those basic parameters most accurately measured, which are as follows:
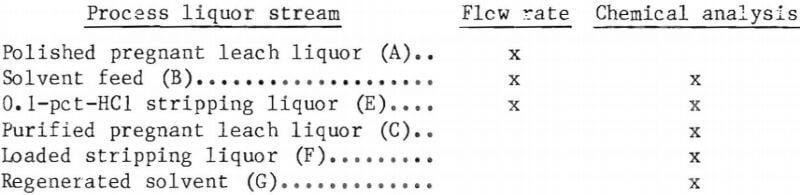
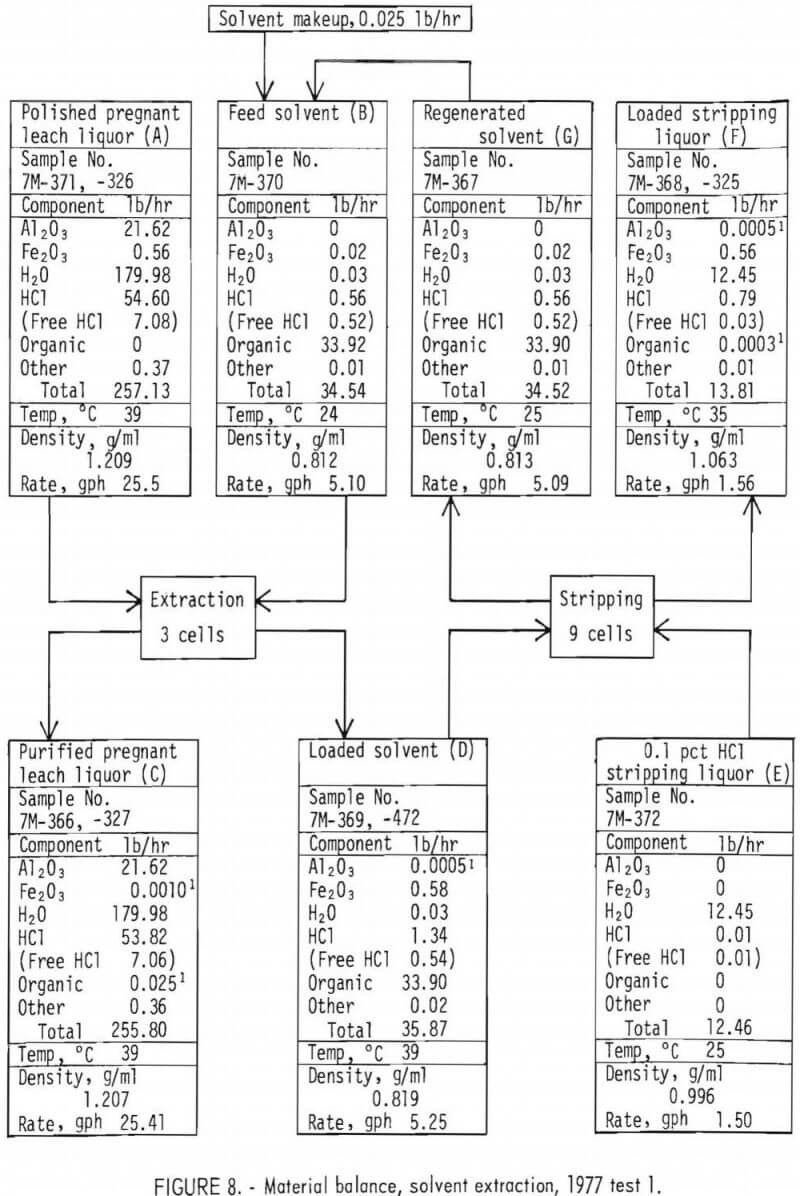
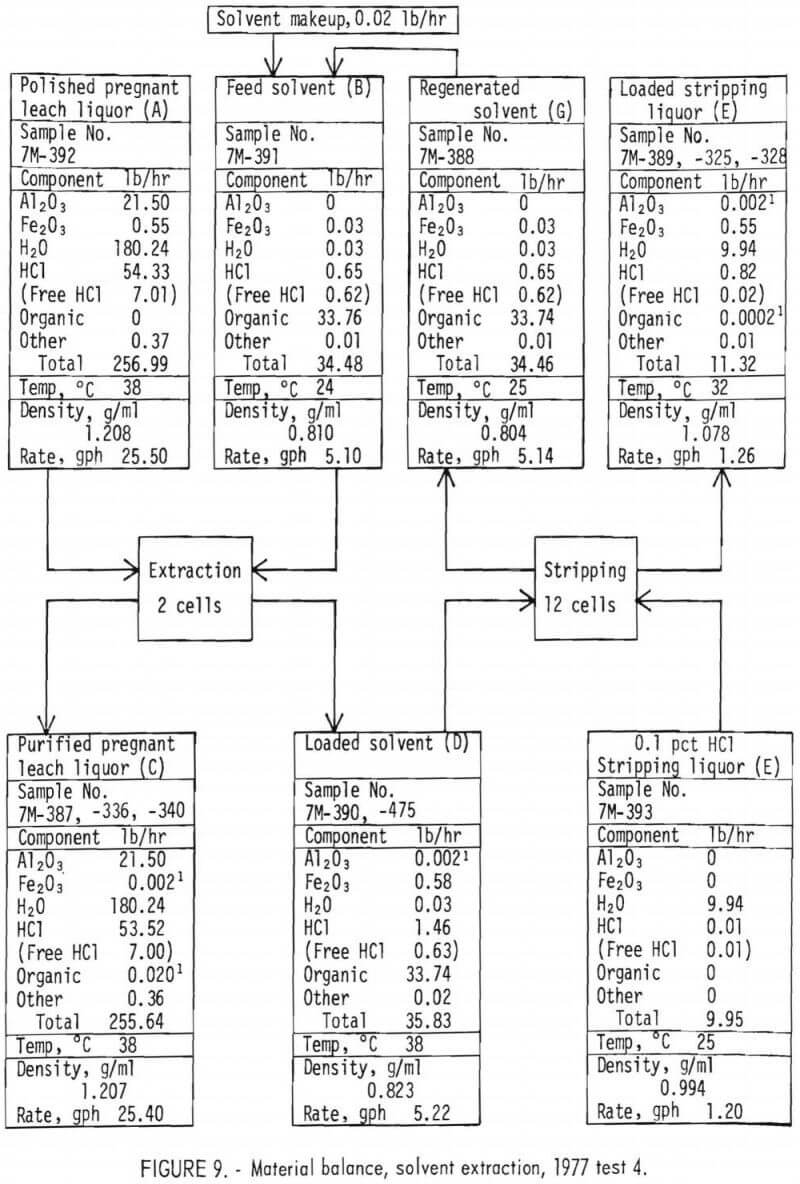
The parameters not used in the balances were either not measured or considered to be less accurate than those used.
Iron Buildup
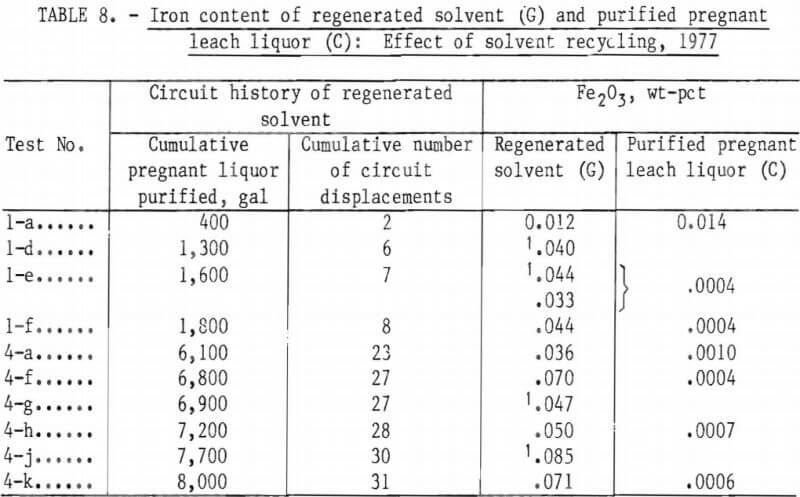
The solvent feed for each test (1-a, 1-b, etc.) was regenerated from the preceding test. Thus, any buildup of iron that could be attributed to continued recycling of the regenerated solvent (G) (as organic feed) could be detected. Despite some analytical variation, iron did not increase above a level of 0.5 to 0.8 pct Fe2O3 in the recycled regenerated solvent (G) as testing progressed. Similarly, the iron content of the purified pregnant leach liquor (C) did not increase as the testing progressed. Table 8 presents the results of Fe2O3 analyses of the regenerated solvent (G) (organic feed) samples, as well as the Fe2O3 analyses of the purified pregnant leach liquor (C) for the individual test increments (1-a, 1-b, etc.) of tests 1 and 4.
Minor Impurities
Detailed analyses of polished pregnant leach liquor (A), carbon-treated purified pregnant leach liquor (I), and loaded stripping liquor (F) are shown in table 9. Nearly every impurity in the pregnant leach liquor, other than iron, was unchanged by the solvent extraction process and activated carbon treatment. The only exceptions-copper, zinc, and gallium-decreased in the purified pregnant leach liquor and appeared concentrated to a degree in the loaded stripping liquor (F).
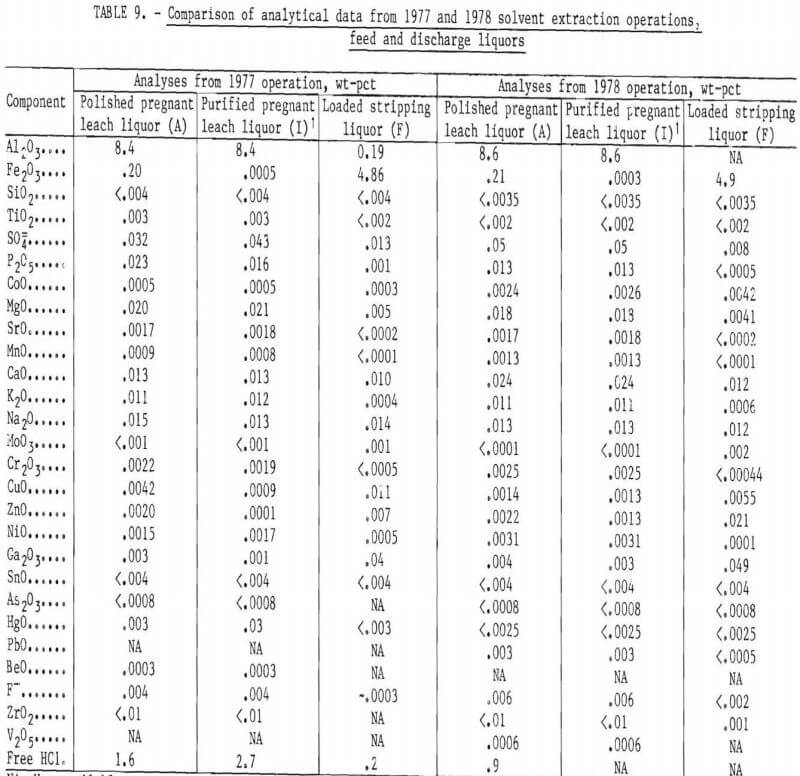
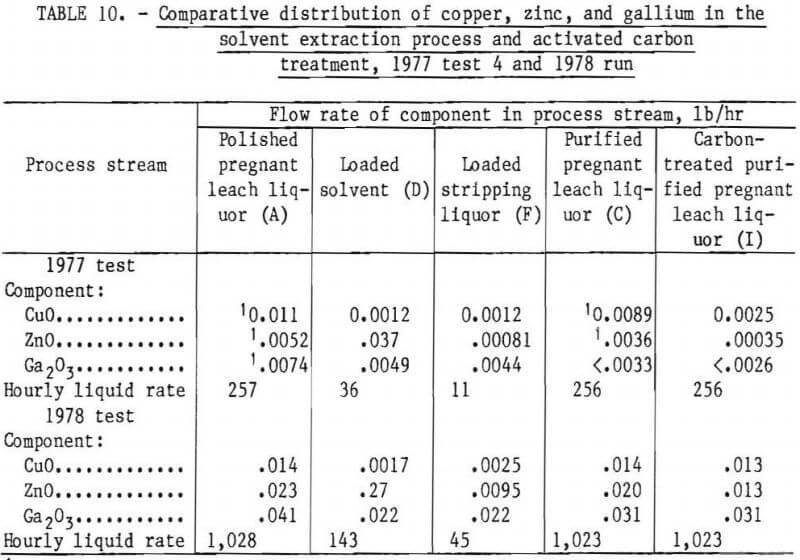
Distributions of copper, zinc, and gallium in the solvent extraction process and activated carbon treatment are compared in table 10 for test 4 of 1977, and also for the 1978 run. The stripping stream effectively removes all the copper and gallium in the loaded solvent stream, but only part of the zinc. Part of the zinc is picked up by the activated carbon. With time, zinc steadily builds up in the loaded solvent stream, but gallium and copper do not, as shown in table 11. At the end of the 1978 run, zinc reached 0.19 pct ZnO in the loaded solvent and only stripped to 0.16 pct. Only about 17,000 gal of purified liquor had been treated at this stage. The zinc content of the solvent phase in the SX cells increased by 0.01 wt-pct ZnO for each 1,000 gal of pregnant leach liquor treated. If this buildup continued unabated, it could present problems for commercial application of this solvent extraction process by lowering the iron-loading capacity of the solvent. However, this zinc buildup might be eliminated by operating the stripping circuit at a lower pH as has been done in certain zinc solvent extraction processes.
On a “bone-dry” basis, the following minor impurities in the purified pregnant liquor would appear to exceed the maximum limited for final product alumina: SiO2, TiO2, P2O5, MgO, MnO, CaO, Cr2O3, CuO, ZnO, NiO, Ga203, and V2O5. Na2O alone fell below the limits. Of the various impurities present in the final product alumina obtained by the thermal decomposition of aluminum chloride hexahydrate (as crystallized from concentrated and purified pregnant leach liquor), only P2O5 and MgO exceeded specifications. As with all the other impurities (except iron, copper, zinc, and gallium), phosphorus and magnesia were unchanged by the solvent extraction circuit. An additional processing step, such as crystallization, will be necessary to reduce the concentration of these two impurities. Maximum limits in the final alumina product for SO4, CoO, SrO, MoO, SnO, As2O3, H2O, PbO, BeO, F-, and ZrO are not available; however, they are not believed to be critical.
Removal of Entrapped Organics
The miniplant operation demonstrated that the major part of the organics entrapped in the purified pregnant leach liquor (C) could be removed by using a cloth-packed coalescer. The operation also demonstrated that nearly complete removal of the more intimately associated organics still remaining could then be achieved by passing the purified pregnant liquor through a column packed with granular activated carbon.
Minimizing Entrainment During Extraction and Stripping.—In 1977, the purified pregnant leach liquor (C) had an average organics content of 74 ppm, with no significant difference caused by circuit feed rate (table 12). The higher rate of feed, 51 gpm, was very close to General Mills recommendation of 1 gpm/sq ft of settler area.
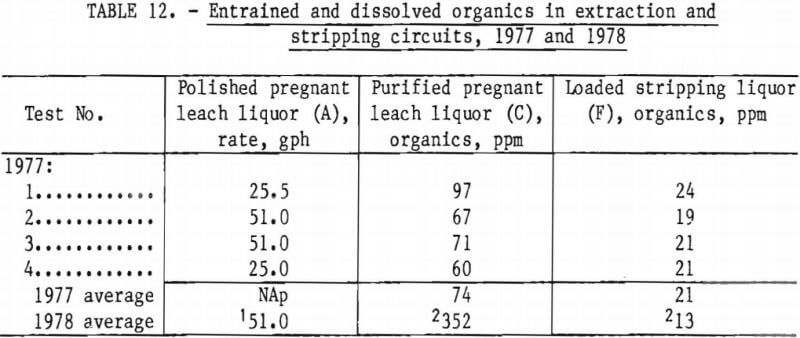
In 1978, however, the organics content of the purified pregnant liquor averaged 352 ppm, most likely because of excessive mixer speeds in the extraction circuit and the presence of 2- to 3-inch emulsified zone visible between the solvent and aqueous phase. The average extraction impeller speed was
9.6 rps in the 1978 run versus 7.1 rps in test 2 in 1977. The turbine factor in the 1978 run was double that in 1977, 72 versus 32. This factor—the product of the turbine speed (rps) cubed, times the turbine diameter (feet) squared—should be below 20 for minimum organics entrainment.
Organics entrainment in waste stripping liquors was lower in 1978 than in 1977, despite an average turbine factor of 37 in 1978 versus 20 in 1977 (test 3).
The effect of the picket fences on entrainment of one phase in another and on the thickness of the mixed phase zone was not established in this study.
Removal of Entrained Solvent Phase by Means of Coalescing.—In 1977, the total organics content of the purified pregnant leach liquor (C) was reduced about tenfold by passage through a skimmer tank (of 3 to 6 hours retention time) and the coalescer. The final product varied between 2.5 and 12 ppm, averaging about 7 ppm. In 1978, the purified pregnant leach liquor (C) averaged 352 ppm organics and was only reduced in the skimmer tanks and the coalescer to 47 ppm (or 24 ppm on the basis of a carbon column material balance). The higher coalescer discharge organics content in 1978 shows that mixers were causing more emulsification than in 1977. Possible remedial steps would be reducing the turbine diameter to one-third of mixer cell diameter, or using pumps for liquor transfer between cells instead of mixer turbines.
Removal of Organics Contamination by Means of an Activated Granular Carbon Treatment.—Columns packed with granular activated carbon lowered the organics content of the purified pregnant liquor to about 0.3 ppm in 1977. Carbon column test CT 3 is summarized in a material balance shown in figure 10 Organics removal was about 97 pct. These low organics contents were attained with only lightly loaded columns; however, extraction would probably decrease
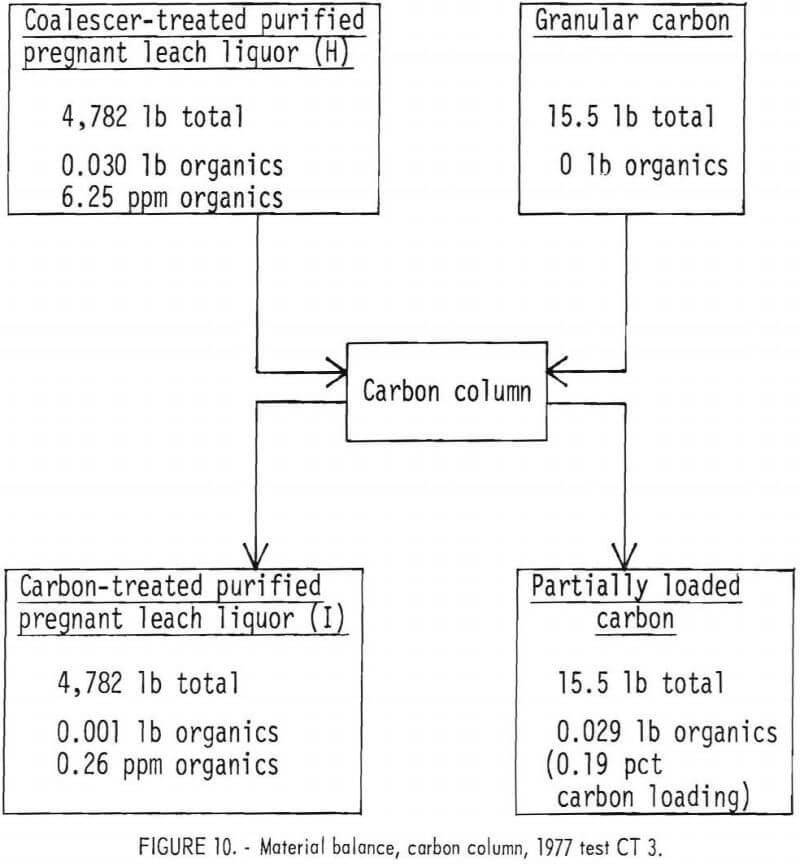
as column loading increased. As the organics-loaded zone travels down the carbon column, the length of the very lightly loaded zone decreases. It is this lightly loaded zone that brings the organics content below that produced from the “mass transfer zone.” Always having some minimum length of the lightly loaded zone could be achieved by running two columns in series. Removal of organics by carbon column was 93 pct in the 1978 run, as shown in the material balance in figure 11. The treated liquor contained 1.7 ppm organics.
Results of analyses of the organics content of the carbon column from 1977 test CT 3 by strata are shown in table 13. The organics content in the top 1¾-inch zone is 11.6 wt-pct; in the next 2-inch zone, 2.8 wt-pct; in the following 2-inch zone, 0.7 wt-pct; and in the bottom 24-inch zone, less than 0.1 wt-pct. Interpretation of these data indicate a practical loading of about 7 or 8 wt-pct for granular carbon. At a 7 wt-pct loading, column CT 3 could have handled a total of about 17,000 gal, instead of the 475 gal actually
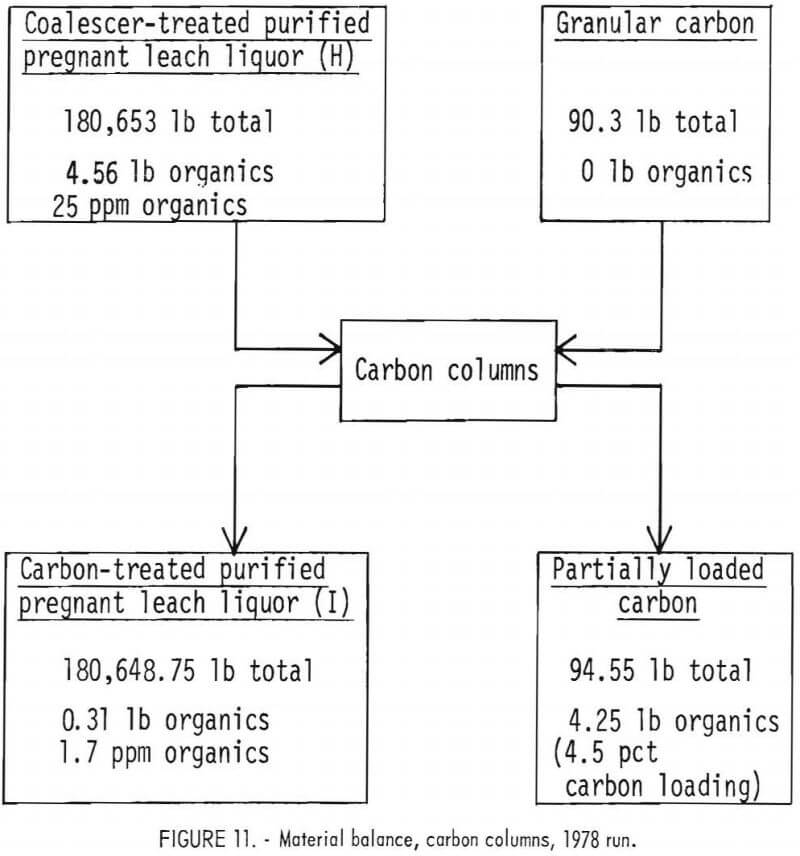
treated. In the 1978 run, the final organics loading of the two parallel carbon columns averaged only about 4.5 wt-pct, which may be maximum loading, because the carbon column discharge that averaged 1.1 ppm organics content over the first 5 days of operation rose over the last 2 days to 3.8 ppm. However, in 1978, the columns were fed by piston-type metering pumps that produced a visible pulsation within the carbon bed. Such movement of the carbon particles may have mixed the loaded and unloaded bedding zones and reduced the loading capacity of the columns.
With a loading of only 7 wt-pct solvent in the carbon, regeneration of the activated carbon bears consideration. However, it was not investigated in this SX study. Organics can usually be removed from activated carbon by thermal treatment in a multiple-hearth furnace at about 1,600° F, often with a steam addition on one of the lower hearths. Steam alone can sometimes be used J. Stephenson, of Pathfinder Mines in Gas Hills, Wyo., reports washing with a 10-pct caustic solution to remove a 5-pct Alamine 336-95-pct kerosene mixture from activated carbon. In this procedure, the used carbon is mixed for 1 hr with 1 bed volume of the caustic solution, then drained and washed twice.
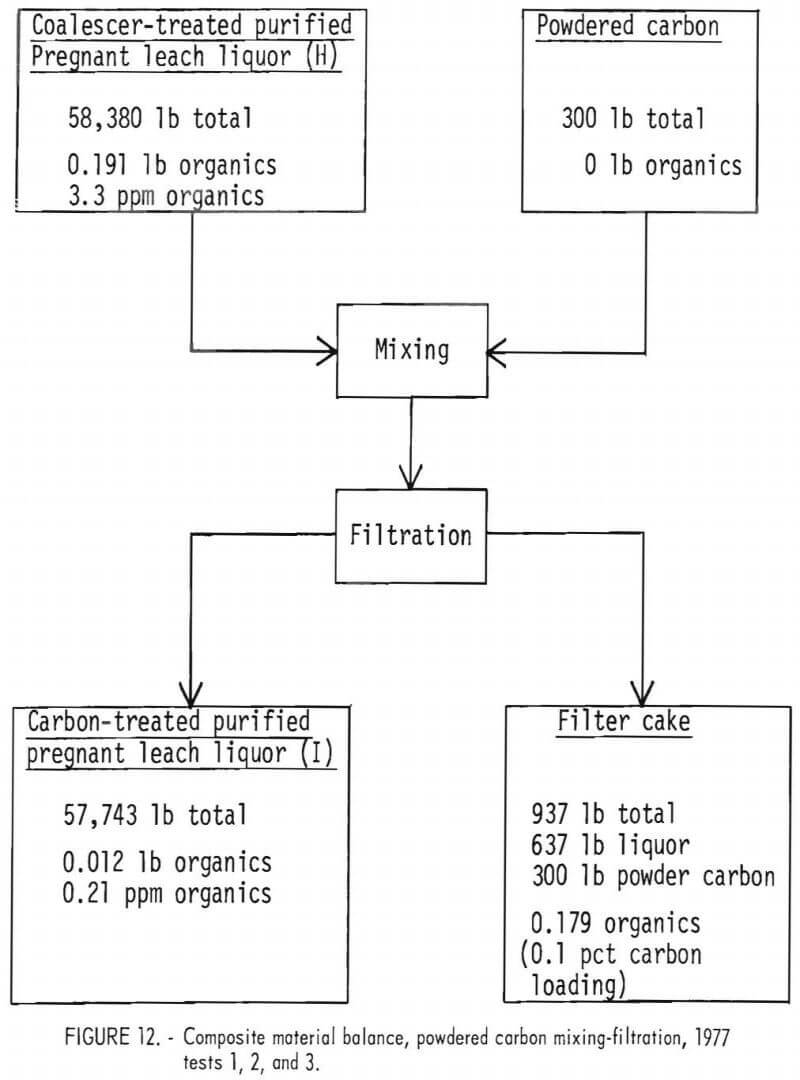
Removal of Organics Contamination by Means of Activated Powdered Carbon Treatment.—Mixing coalescer-treated purified pregnant leach liquor with powdered activated carbon lowered the organics content of the liquor to 0.2 ppm. Three separate mixing-filtration tests summarized in figure 12 show that removal of organics averaged about 94 pct; however, powdered carbon loading was only about 0.1 pct. This very low loading occurred because the feed liquor from the coalescer had been expected to contain 50 ppm organic matter, whereas the feed actually contained only 3 to 4 ppm organic matter. Optimum carbon usage and loading was not determined and would require further study.
Conclusions
An engineering study of the removal of iron from pregnant leach liquor by solvent extraction, produced by leaching calcined kaolinitic clay with hydrochloric acid, has been completed. All major objectives of this study have been accomplished.
Pregnant aluminum chloride leach liquor was purified by solvent extraction to an iron content of 0.0006 wt-pct Fe2O3. The solvent contained a mixture of 15 vol-pct Alamine 336, 10 vol-pct decanol, and 75 vol-pct kerosene. The loaded stripping liquor produced by regeneration of the solvent contained 4.5 to 4.9 wt-pct iron as Fe2O3, well above the targeted level of 4.2 wt-pct Fe2O3. Organics in the pregnant leach liquor purified in the solvent extraction circuit were lowered by skimming, followed by coalescer and activated carbon treatment, to between 0.2 and 1.7 ppm.
Material balances and engineering characteristics of iron removal and solvent regeneration in the SX circuit were sufficiently quantified in this work for subsequent scaleup and cost studies. Both extraction and stripping circuits were studied at several feed rates, phase ratios, and number of mixer-settler units.
Other than iron, only copper, zinc, and gallium showed any evidence of extraction by the solvent phase. Of these, only zinc appeared to concentrate in the solvent phase during solvent extraction. The zinc content in the regenerated solvent reached 0.16 wt-pct ZnO after purification of about 17,000 gal of pregnant liquor.
Some removal of copper, zinc, and gallium from purified pregnant leach liquor by activated carbon treatment appears likely, but actual percentages have not yet been established.
