 Pure rhenium is a refractory metal with a very high melting point of 3180°C. Extraction of rhenium from molybdenite concentrates and other primary sources has been studied for many years. Hydrometallurgical methods using Br2, HNO3, Ca(OH)2, NaOH or Na2CO3 have been investigated. Recently, a new process using halogen salts and ammonium as well as oxygen for recovery of platinum group metals and rhenium was developed. This new process employs iodide/iodine and/or other halogen salts to effectively extract platinum group metals and rhenium from spent automobile exhaust catalysts and petroleum refining catalysts.
Pure rhenium is a refractory metal with a very high melting point of 3180°C. Extraction of rhenium from molybdenite concentrates and other primary sources has been studied for many years. Hydrometallurgical methods using Br2, HNO3, Ca(OH)2, NaOH or Na2CO3 have been investigated. Recently, a new process using halogen salts and ammonium as well as oxygen for recovery of platinum group metals and rhenium was developed. This new process employs iodide/iodine and/or other halogen salts to effectively extract platinum group metals and rhenium from spent automobile exhaust catalysts and petroleum refining catalysts.
Usually rhenium used in catalysts is in metallic form. It disperses in catalyst matrix as very fine particles. Spent petroleum refining catalysts contain up to about 2500 ppm of rhenium together with a substantial amount of platinum. The new process using halogen salts is based on the fact that platinum group metals and rhenium metal are soluble in leaching solutions containing halogen salts and ammonia under appropriate conditions. However, the fundamentals of this new process are not fully understood.
The leaching behavior of gold and platinum in iodide/iodine solutions has been studied.
However, the dissolution behavior and leaching mechanism of rhenium in aqueous solutions has not been investigated in detail. The current investigation is to understand the leaching mechanism of rhenium in ammonium iodide and iodine solutions.
Batch leaching experiments were carried out in a 1000 ml autoclave made by Parr Instrument Co. The autoclave was equipped with an impeller stirring device. The temperature was controlled within ±3°C and the stirring speed was controlled. One piece of high-purity (99.98%) rhenium square plate with total surface area of 12.5 cm² was used in each leaching experiment. The rhenium plate was suspended in the leach liquor when the experiment was carried out.
Leaching solution of 500 ml was used in each experiment and was prepared by dissolving known amounts of reagent grade chemicals in distilled water. Before leaching experiment was conducted, polished rhenium plate and leaching solution were placed in the autoclave. The autoclave was sealed and heated to a desired temperature. The leaching time was taken into account when the desired temperature was reached. About 10 ml liquid samples were withdrawn at various time intervals.
The rhenium concentration of the solution sample was analyzed by a UV spectrophotometer at the wavelength of approximate 420 nm. The analytical method adopted was based on the literature information.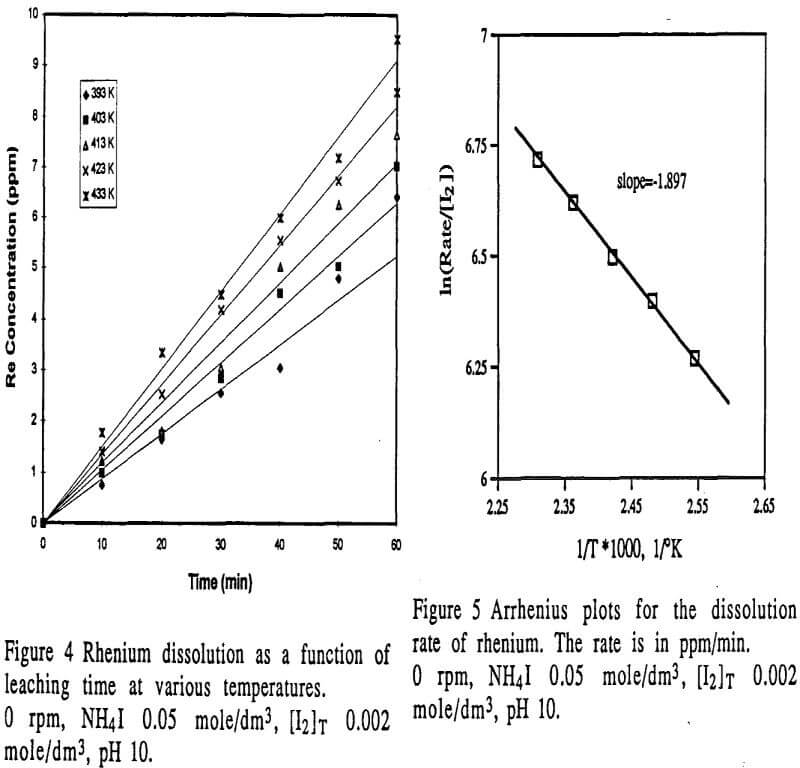
In general, leaching experiments were carried out at 160°C, with a specified stirring speed, and lasted for 60 minutes. The standard error of leaching experiments was less than 4%.
According to the thermodynamics discussed in the previous section, rhenium can dissolve in iodide solutions in the presence of an oxidant even at room temperature. A leaching experiment was conducted at room temperature and found that after 117 hour leaching, rhenium dissolution into the solution was not found to be substantial. The concentration of rhenium was only 32.6 ppm. This means that the dissolution of rhenium at ambient temperature is very slow. In order to obtain more practical information on the leaching behavior of rhenium, subsequent experiments were carried out at elevated temperatures, in an autoclave. Ammonium iodide was used in the leaching solutions. It is noted that the pH of ammonium solution can be easily maintained because of its buffering characteristics.
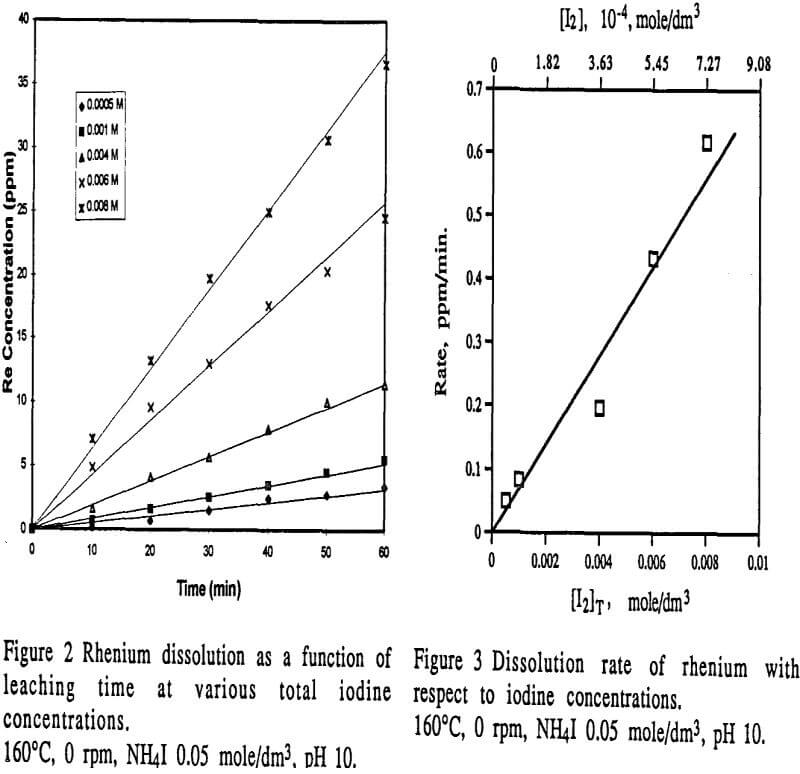
Figure 2 shows a set of typical leaching results of rhenium as a function of leaching time. From the figure, it can be seen that during 60 minute leaching time, rhenium dissolution exhibited a linear correlation with leaching time. This leaching behavior can be described by Equation 6. Therefore, the dissolution rate of rhenium can be obtained from the slope of experimental data plots as shown in Figure 2.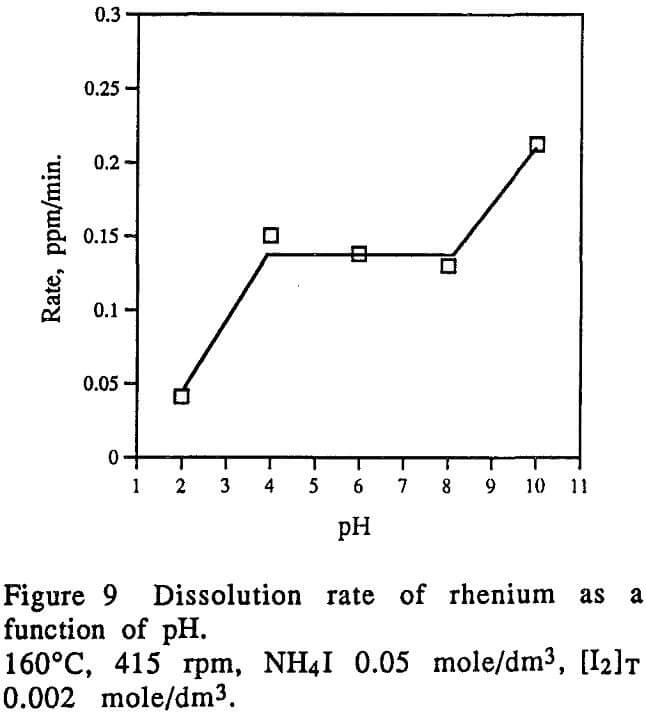
- Effect of iodine concentration
- Effect of pH of iodide solution
- Effect of iodide concentration
- The thermodynamic analysis and experiments indicate that rhenium can dissolve in iodide/iodine solutions even at ambient temperature. However, the dissolution was facilitated at elevated temperatures. The dissolution of rhenium in ammonium iodide/iodine solutions can be described by means of a kinetic model under the conditions studied.
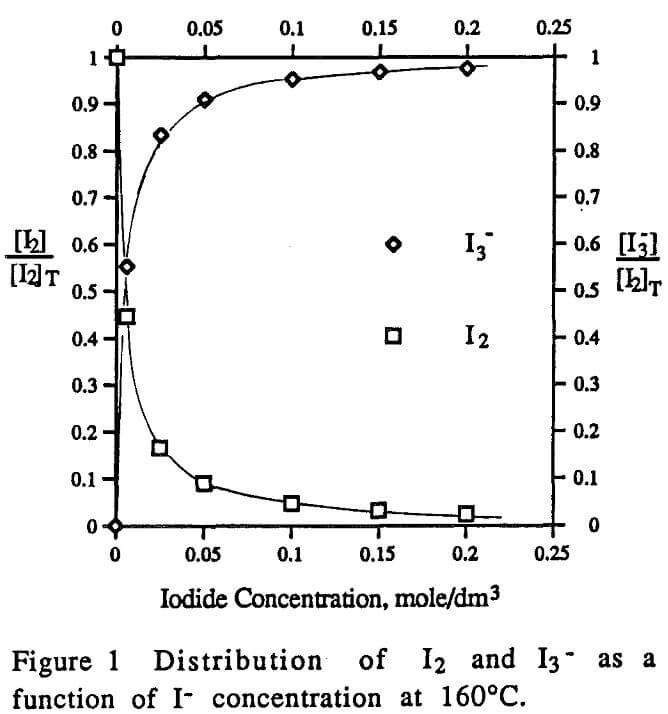
- The reaction of rhenium with iodine, not triiodide, was the principal reaction governing the leaching of rhenium in iodide/iodine solutions.
- The dissolution of rhenium was controlled by mass transfer of iodine with an apparent activation energy of 15.77 kJ/mole.
- Iodide concentration has negative effect on the dissolution rate of rhenium. The pH of the leaching solutions also has a significant effect on the dissolution rate of rhenium. High pHs will benefit the leaching of rhenium.

