Table of Contents
The Bureau of Mines is conducting research to develop cost effective, environmentally compatible advanced mining technologies for the selective extraction of critical and strategic metals. Critical and strategic metals (e.g. manganese, cobalt, and chromium) are essential to domestic steel, aerospace, electronic and defense-oriented industries. The United States no longer has high-grade deposits of these minerals and must import most of its supply from foreign sources (Dept. of Interior, 1990). Advanced mining technologies such as in situ leach mining (ISLM) could make domestic production of these metals feasible for ore deposits that are now unattractive to conventional mining because of their small size, low grade, or depth.
In situ leach mining requires lower capital investment, manpower requirements, and energy consumption than does conventional mining and milling. Therefore, in situ leach mining may be economically attractive for many critical and strategic metal deposits. In situ leach mining offers improved health and safety conditions for the miner and may be performed at substantially reduced environmental impact. Based upon criteria which include analysis of chemical and metallurgical characteristics, mineralogy, and deposit geology, the Bureau has determined that manganese is one critical and strategic commodity with potential for recovery using in situ leach mining techniques.
Manganese is a strategic commodity because of its importance in steel production. In 1989, 100 % of the manganese consumed in the United States was imported (Dept. of Interior, 1990). Industry has not been able to employ conventional mining techniques to recover manganese since the late 1940’s, because of the prohibitive costs of mining and processing low grade, relatively small, and deep ore deposits. The United States has low grade deposits of manganese that could be utilized to reduce the reliance on foreign sources of supply, such as Artillery Peak in Arizona, the Cuyuna Range in Minnesota, and the Aroostook District in Maine. Previous research shows that manganese can be extracted from low grade domestic ores by leaching with aqueous sulfur dioxide (SO2) solutions, but it is unknown whether this technique can be used to develop an overall in situ leach mining process. Therefore, the Bureau has initiated a new research program to study the application of in situ leach mining techniques to recover manganese from domestic deposits. This report presents the Bureau’s experimental approach to this problem and preliminary findings from this research.
Previous In Situ Leach Mining Research by the Bureau of Mines
The Bureau of Mines has gained significant experience in the area of in situ leach mining (ISLM) research from its past and current projects on uranium and copper ores. The first successful application of ISLM in which the Bureau of Mines participated, was uranium leach mining of sandstones in Texas (Larson, 1978). More recently, the Bureau has concentrated on ISLM of a deep copper oxide deposit in Arizona. This project has demonstrated the importance of geologic characterization studies in predicting metal recoveries and in determining the physical and chemical constraints for in situ leach mining operations (Paulson et al., 1987; Paulson, 1989; Cook and Paulson, 1988; Cook, 1988; Paulson and Kuhlman, 1989).
ISLM has been found to be most effective when the commodity of interest can be selectively removed from the host ore. Selective leaching can be achieved in a variety of ways. The lixiviant can be a non-specific solvent such as acid which will react to dissolve both ore and gangue. In this type of system, selective leaching can be effective if the amount of acid contacting the ore minerals is much greater than that contacting gangue. An example of this situation is in fracture hosted porphyry copper deposits where flow channels are lined with copper bearing minerals. Selective leaching can also be achieved with a non-specific solvent if the ore dissolution reactions are much faster than the gangue dissolution reactions. An example of this is a copper oxide ore deposit where copper oxide minerals are much more soluble in dilute sulfuric acid than matrix gangue minerals such as quartz or feldspar.
Typically, manganese deposits do not have the required characteristics to be leached by a non-specific lixiviant. Oftentimes, manganese minerals are intimately associated with iron oxide minerals as part of the rock matrix and are not associated with fractures. Because of these characteristics effective leaching can only be achieved by lixiviant systems that are chemically selective for manganese in the presence of gangue minerals. Chemically selective techniques developed for manganese may also be applicable to other commodities in the future.
Previous Research on Manganese Ore Leaching
Much research has been reported on the leaching of manganese ores and on individual manganese minerals. Previous research that directly pertains to mining or mineral processing of manganese may also have application to in situ leach mining of manganese.
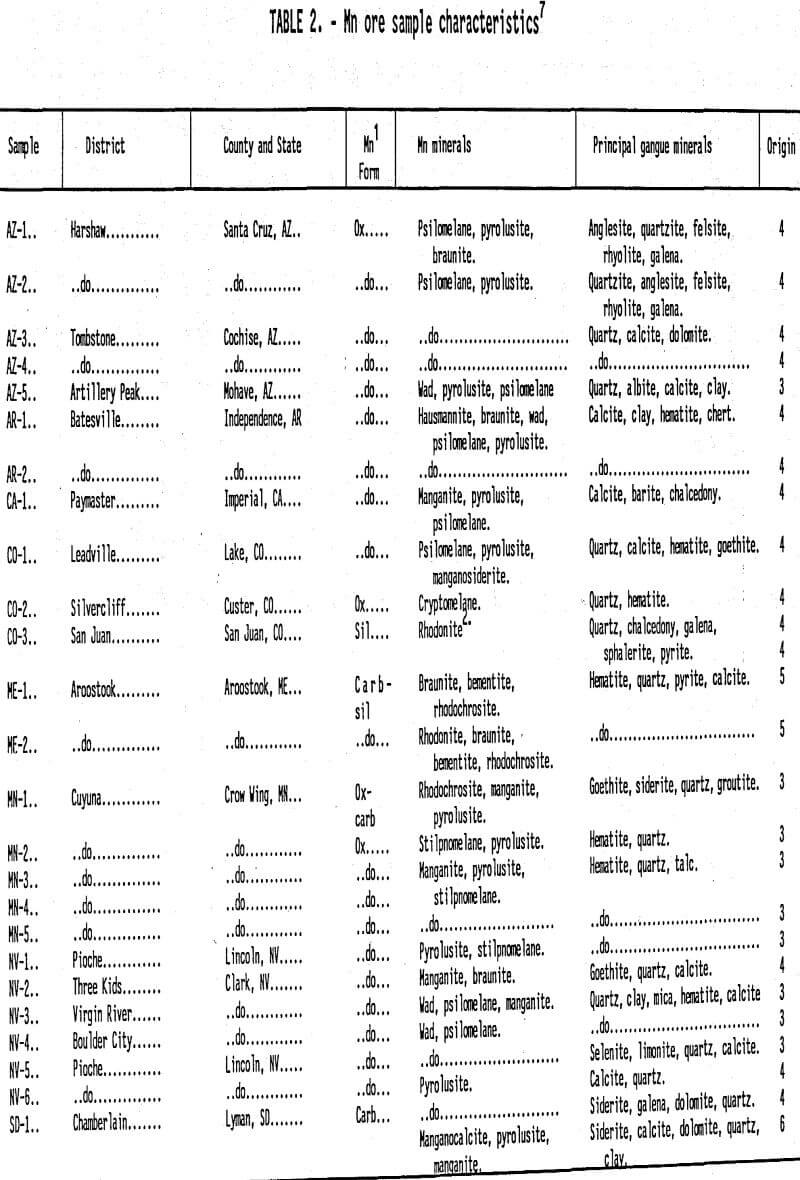
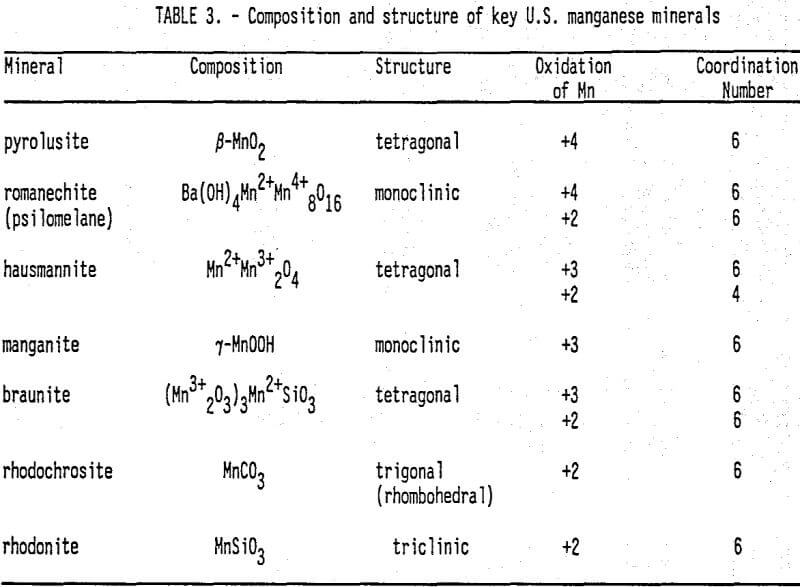
Domestic Mn Deposits and Their Leachabllity: A comprehensive discussion of worldwide manganese deposits, including U.S. deposits, is presented by Roy (1981). Potter et al. (1982) reported six U.S. locations with significant low grade manganese ore deposits. Pahlman and Khalafalla (1988) reported on SO2 leaching of manganese ores, including a number of samples from the above six deposits (Table 1). In addition to manganese mineralogy, Table 2 lists the principal gangue mineralogy. Gangue consumption of lixiviant is an important consideration during in situ leach mining optimization as it depletes the lixiviant available for metal dissolution. Oxides are the most common and abundant manganese minerals found in U.S. deposits, followed by carbonates and silicates. Table 3 summarizes the composition and structure of the key manganese minerals found in U.S. manganese deposits, including the valence and coordination number of manganese metal centers.
From a geochemical kinetics perspective, there are several observations to be made from the data in Table 3. First, these minerals have manganese centers With a range of oxidation states (4+, 3+, 2+). The charge on a manganese center significantly affects the chemical stability and dissolution reactivity of the mineral. Second, manganese centers usually have a bonding coordination number of six, indicating the octahedral bonding symmetry common to manganese ions in solution. Third, oxygen is the most common element bonded to manganese. Fourth, the structural differences of manganese minerals vary enough to possibly affect dissolution reactivity. For example, the basic unit for pyrolusite and manganite, form relatively tight chain structures, whereas romanechite consists of triple chains of units that form open tunnels (Burns and Burns 1979). This means that more romanechite Mn sites may be available for dissolution by the lixiviant than pyrolusite or manganite manganese sites.
Selective Oxide Dissolution with Dissolved SO2: One lixiviant, sulfur dioxide (SO2), has been extensively investigated since it is capable of rapidly dissolving acid resistant manganese oxides such as pyrolusite. The Bureau of Mines has a long involvement in the processing of low-grade manganese ores with SO2 solutions. Early Bureau work was reviewed in a Bureau Information Circular by Dean, Leaver, and Joseph (1934), which detailed hydrometallurgical processes using SO2. Wyman and Ravitz (1947) discussed the selective nature of SO2 leaching for manganese over iron and other gangue in domestic ores. They determined the composition of the final leachates, but did not construct detailed composition versus time (or SO2 amount) leaching curves.
Back, Ravitz, and Tame (1952) reported SO2 processing of MnO2 ores under aerobic conditions. They observed decreased S2O6= formation with lowered pH and increased agitation (i.e. increased solution oxygenation). For example, only 3% S2O6= was formed at pH 0.75 versus 8 % at pH 1.90 for well agitated systems. This work was part of the research for the “dithionate process” that was developed by the Bureau for processing low-grade manganese dioxide ores.
Herring and Ravitz (1965) found that the rate of manganese dioxide reductive dissolution was faster at lower pH values, for example, 7.6 micromole min-¹ (cm²)-¹ at pH 1.82 versus 80.4 micromole min-¹ (cm²)-¹ at pH 1.09. These results indicated that manganese dissolution was approximately 10 times faster for SO2 than for HSO3-. The reaction rates were also faster with increasing stirring rate, indicating a transport-controlled reaction. This was confirmed by a low apparent activation energy of 4.5 ± 0.2 kcal/mole.
Henn, Kirby, and Norman (1968) reviewed 10 major processes for manganese ore which employ SO2. Nine of the processes use SO2, H2SO4, or a combination of SO2 and H2SO4. The tenth process uses a SO2 roast at 600° to 850° C before a water leach.
Miller and Wan (1983) reported work devoted to the kinetics of reductive dissolution of electrolytic MnO2 and pyrolusite. They concluded that the leaching process was rate-limited by a mineral surface reduction reaction obeying the following rate law:
Rate = d(MnO2)/dt = kxKa0.5Ac [SO2]total0.5 ([H+]/([H+]+ Ka))0.5……………………………(1)
where kx = heterogeneous rate constant
ka = equilibrium constant for SO2+
H2O = H+ + HSO3-
Ac = active surface area of MnO2 mineral
Dixit and Raisoni have reported their work in two recent reports. In the first report (1987), they demonstrated that sufficiently high concentrations of dissolved O2 will suppress the reductive dissolution of MnO2 with SO2. Viewed from a geochemical perspective, this is a reminder that reductive dissolution of MnO2 requires an overall reducing solution environment, i.e. a low Eh value. In their second report (Raisoni and Dixit, 1988), dissolution rates of MnO2 fit the metallurgical equation for diffusion-controlled release of Mn²+ through an unreacted product layer:
Dissolution Rate = kt = 1 – 2/3 a – (1 – a)2/3…………………………………..(2)
where k = rate constant
t = time
a = fraction manganese leached
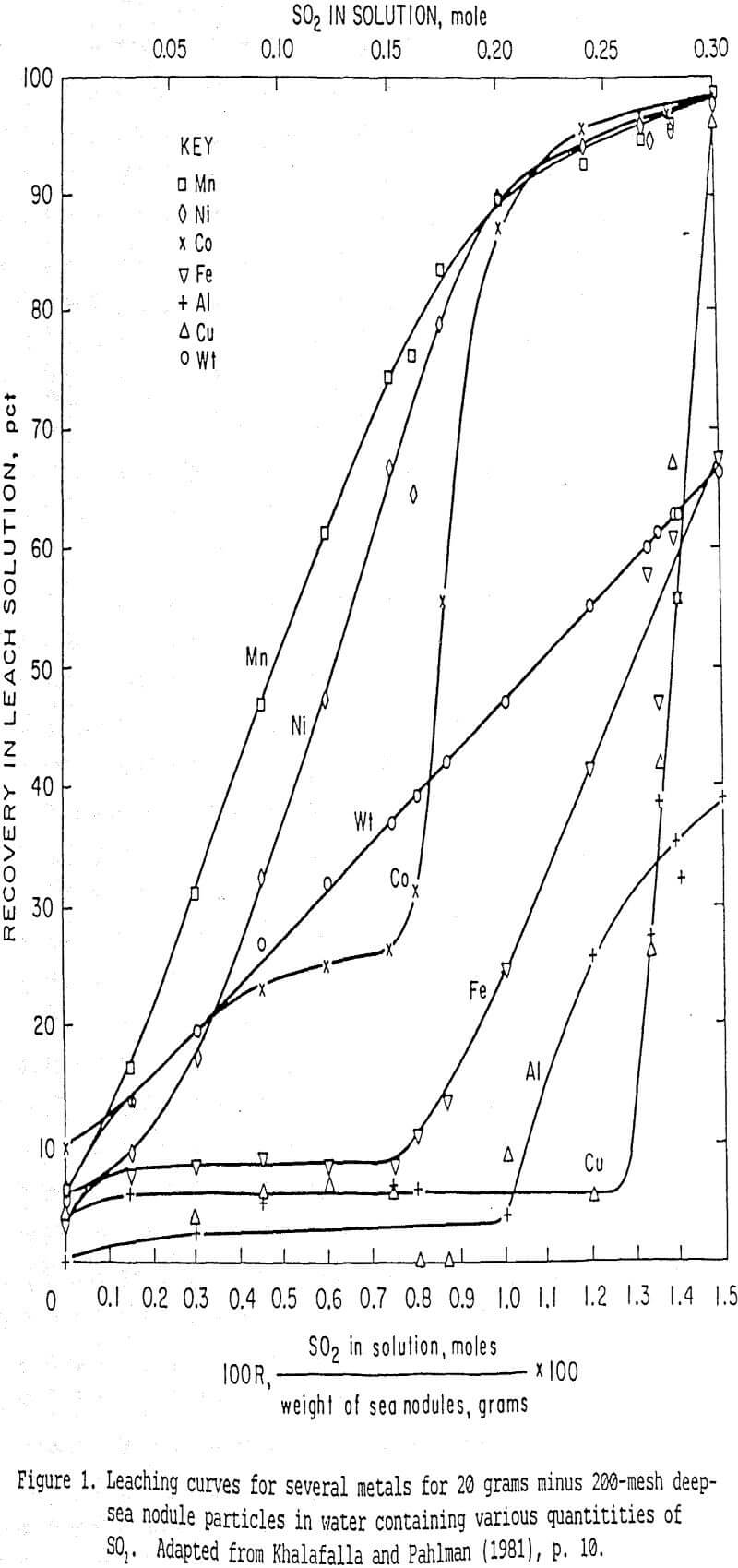
Khalafalla and Pahlman (1981) reported an important Bureau study that demonstrated the use of SO2 for selective extraction of critical and strategic metals (i.e. Mn, Co, Ni) from Pacific ferromanganese nodules. For a series of batch leaching experiments, they observed that increasing molar ratios of SO2 in the lixiviant to grams of nodules leached resulted in a reproducible sequence of different metals being preferentially leached:

Figure 1, from Khalafalla and Pahlman (1981), shows their results and demonstrates the dramatic selective preference of SO2 for Mn, then Ni and Co. Fe, Al, and Cu were leached only after most of the Mn, Ni, and Co had been leached.
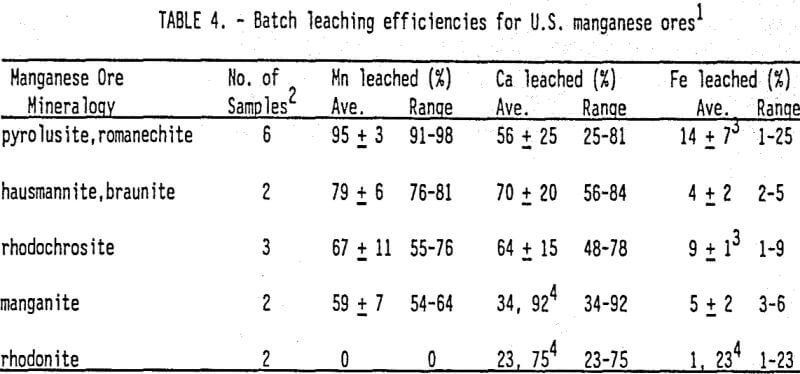
Of the many research reports dealing with the rapid SO2 dissolution of domestic manganese ores, the report by Pahlman and Khalafalla (1988) is the most relevant to in situ leach mining. They presented very encouraging new results from batch and column leaching of 25 U.S. manganese ores exposed to 5-6.4 wt % SO2 solutions. Table 4 summarizes the batch leaching results. First, they achieved typically greater than 90% Mn recoveries for manganese oxides while solubilizing less than 10% of Fe from hematite (Fe2O3), a key gangue mineral. Less abundant Fe carbonate (siderite) and hydroxide (goethite) readily dissolved in SO2 solution. Second, at low SO2 application rates (1 mL/min) to the column, calcite (CaCO3) solubilization was minimized by formation of an insoluble reaction layer, which suppressed further leaching of CaCO3. The reaction layer was composed of a Ca-S compound, most likely gypsum. Lastly and very importantly, the SO2 lixiviant was still effective in leaching Mn from ores with permeabilities as low as 10-² to 10 -4 darcies. This result strongly supports the argument that rapid dissolution of Mn minerals may increase permeability and thus, increase lixiviant penetration into the ore body.
Other Lixiviants for Manganese Ore Dissolution: In addition to SO2 leaching of manganese oxides, other chemical lixiviants have been used to leach manganese minerals. Some of these metallurgical reductants may be adaptable to an in situ leach mining process.
Warren and Devuysst (1972) reported hydrazine hydrate (N2H4.H2O) extracted 90% of Mn from minus 65, plus 150 mesh pyrolusite in one hour. Hydrazine hydrate is a strong reductant but its high cost at the time prevented its consideration for commercial use.
Malati, Rophael, and Bhayat (1981) used N2H4.H2O, followed by pyrophosphate (H2P2O7), to reduce electrolytic -γ-MnO2 and β-MnO2. They also reported that the reduction product of the Mn oxides with N2H4·H2O was Mn³+OOH.
After investigating a number of strong reducing agents, Usatenko and Ryl’kova (1983) reported that potassium iodide (KI) was the most effective reductanf for pyrolusite and romanechite in manganese ores. Using 20 wt. % KI in 0.3-0.4 M acetic acid, they dissolved 96 to 99 % of the pyrolusite and romanechite present. They also suppressed reduction of manganite by adding EDTA to prevent the further reduction of Mn³+ metal centers in manganite to water-soluble Mn²+.
Dresler (1984) reported very rapid leaching of γ-MnO2 with nitrous acid (HNO2) solutions. Since his stirred reaction cell was similar to Herring and Ravitz’s SO2 leaching cell for γ-MnO2, Dresler compared overall leaching rates between his HNO2 work and their SO2 research. The overall leaching rates for HNO2 were reported to be “slightly higher than those in sulfurous acid”. It should be noted that NO2- has the same electron configuration as SO2. Therefore, HNO2 could be a promising potential lixiviant for in situ leach mining if the costs to produce nitrous acid were reasonable.
Recently, Dobos (1988) reported the effective use of a strong-acid cation exchange resin to accelerate acid leaching of metal carbonates such as rhodochrosite (MnCO3) and recover the resulting cation. The resin was added to a sulfuric acid suspension containing 19 wt. % manganese ores. Optimization of the process was performed for such variables as temperature, pH, and supporting electrolyte (i.e. NaCl). Kinetic data was presented.
Selective Leaching of Manganese by SO2 in the Presence of Gangue: An important aspect or advanced mining research is to develop leaching reagents that will selectively leach the target element (i.e. manganese) with a minimum of gangue dissolution. This ability is significant because selective mobilization will reduce processing of in situ mining leachant and will minimize the potential environmental impact of mining. In order to optimize the selectivity of a leach mining lixiviant system, it is necessary to have a detailed knowledge of the geochemistry of target and gangue minerals.
The Bureau of Mines is developing an approach to selective lixiviant development that centers on the geology and geochemistry of target ore bodies. It is called geochemical characterization to emphasize its close relationship to the geologic characterization (i.e. petrology) of a target ore body. Figure 2 summarizes this interactive lab experimentation and geochemical modeling process that begins with ore petrology, proceeds through basic
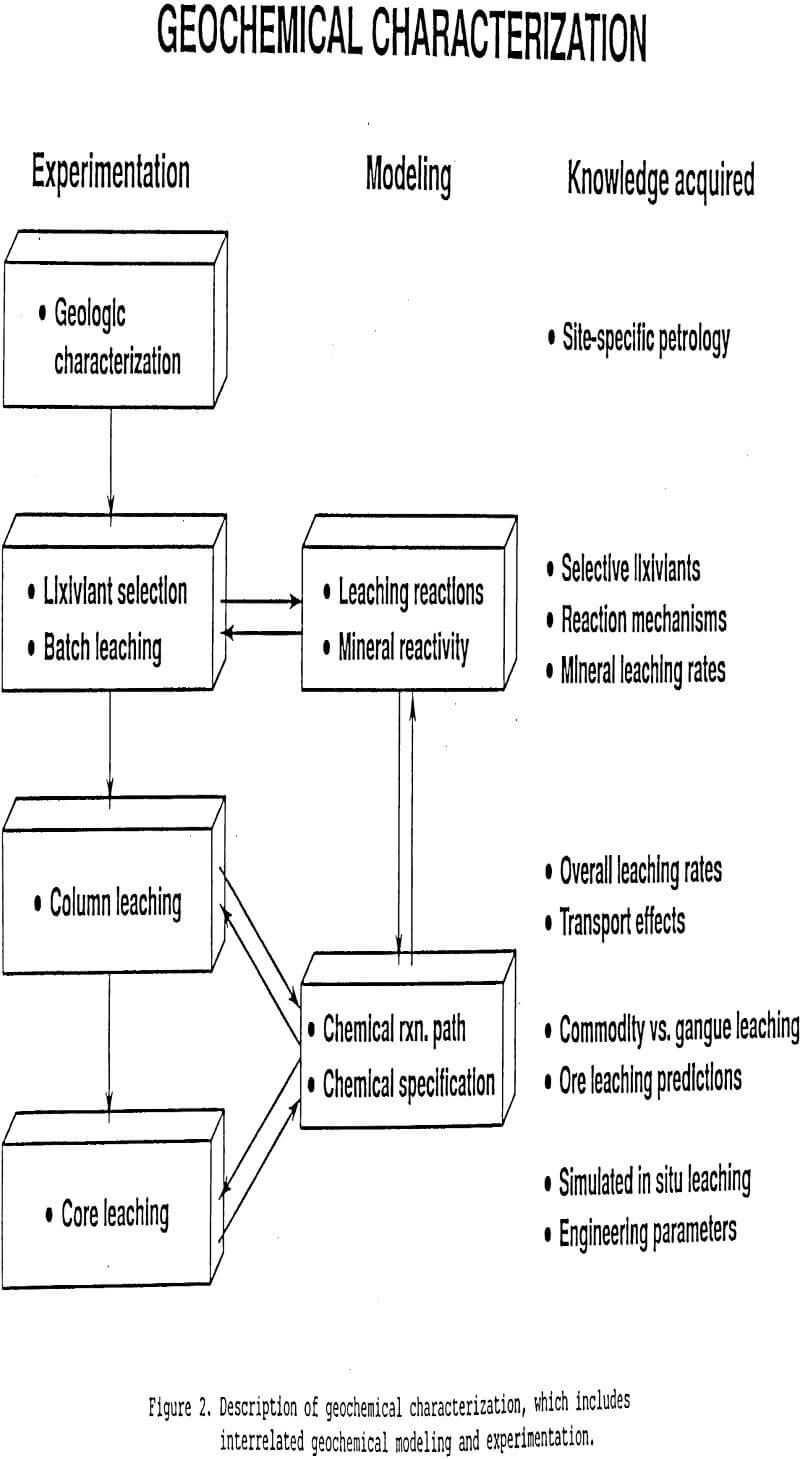
single mineral leaching chemistry, and concludes with generation of design parameters for in situ leach mining.
It is important to emphasize that both a thermodynamic and kinetic study of mineral leaching is necessary because a number of geochemical processes, including mineral leaching, are frequently not at equilibrium or steady-state (Hoffman 1981, Morgan 1989). When ore leaching is not at equilibrium, the relative rates of mobilization of target metals versus gangue will depend on the relative dissolution rates of the minerals. Then the objective is to find a lixiviant system that rapidly dissolves target minerals and slowly dissolves gangue.
In the case of manganese, SO2 has been shown to dissolve the abundant manganese oxides more rapidly than iron oxides, such as hematite (Fe2O3) (Pahlman and Khalafalla 1988). In fact, manganese oxides with Mn 4+ (e.g. pyrolusite, romanechite) dissolve more rapidly than Mn oxides (e.g. hausmannite, manganite).
A recent report by Petrie (1991) has provided a theoretical chemical explanation why Mn4+ minerals would react more rapidly with SO2 than Mn³+ minerals. Manganese is most stable in water as Mn²+ ions. This means that dissolution of Mn4+ and Mn³+ minerals must involve reduction of the manganese to Mn²+. Based on a molecular orbital treatment of manganese mineral reduction by SO2, reduction of Mn4+ minerals (e.g. pyrolusite) should occur more rapidly than Mn minerals (e.g. manganite). Further geochemical characterization is currently underway to further identify the leaching kinetics of manganese and gangue minerals present in key domestic manganese ore bodies.
Experimental Approach
The Bureau of Mines has been conducting in situ leach mining research since the 1970’s. While this research has covered a number of different areas, perhaps the most important facet of this research has been to combine laboratory leaching (i.e. geochemical characterization) with geologic characterization studies. Information generated from these studies is essential to developing an understanding of the reaction mechanisms operating during leaching and in assessing a number of variables which impact on the overall process. The ultimate goal of such laboratory studies is to formulate predictions and estimates of what can be expected during actual in situ leach mining of candidate deposits.
Previous Bureau of Mines in situ mining research (BuMines 1981, BuMines 1989, and references therein) has led to the development of a systematic experimental approach which combines a variety of laboratory leaching experiments with detailed pre- and post-leach petrologic studies. This approach allows the study of ore leaching characteristics with respect to of variables such as ore and gangue mineralogy, textural features, and lixiviant composition. This information is then combined with deposit-specific data obtained from field studies to evaluate the potential for in situ leach mining of that particular deposit. In addition to laboratory research to study the leaching process, another very important part of the laboratory research is an evaluation of the various metal recovery options which are both technically and economically feasible. A viable metal recovery scheme is critical, because without it in situ leach mining will be unsuccessful, regardless of favorable leaching and field conditions. As part of the Bureau’s critical and strategic minerals in situ mining program, a number of different domestic manganese deposits are being evaluated for their amenability to in situ leach mining. The following sections describe the types of laboratory and field studies that are being conducted to facilitate these evaluations.
Geochemical Characterization – Leaching Experiments
A variety of leaching experiments are conducted in order to determine the leaching characteristics of each ore type being evaluated. These experiments include batch, cube, column, and core leaching tests. It is important to closely control and monitor the experiments, since SO2 is volatile and will be lost from an open system, thus complicating the interpretation of leaching results. Batch leaching tests are usually conducted on crushed or finely ground ore which are continuously agitated and are intended to determine the bulk reactivity of the ore and also yield information on kinetics of leaching. The batch tests are used to investigate the response of particular lithologies or mineral assemblages to the lixiviant. The batch experiments allow precise control and measurement of system variables such as pH, Eh, temperature, and SO2 concentration in a closed system. The effects of modified lixiviants on leaching behavior can be quickly determined in batch tests. They are also useful for estimating maximum metal recoveries, recovery rates, and SO2 consumption by gangue minerals. Cube leaching experiments are conducted-by suspending a cube of ore in a continuously-agitated SO2 solution, and are intended to determine how the leaching front advances and at what rate. These tests often illustrate the textural influences of the ore on leaching.
Once a basic understanding of the leaching characteristics is obtained through the batch and cube leaching studies, column and core leaching experiments are conducted to develop estimates of parameters such as Mn recovery, recovery rates, gangue reagent consumption, and permeability changes expected during true in situ mining. Column leaching tests using crushed ore are conducted at different scales depending on ore availability and leachant requirements. It is ideal to use a closed system for the column tests in order to avoid SO2 volatilization prior to application to the columns or after collection of the Mn-bearing leachant if any unreacted SO2 remains in solution (Figure 3). Compressed SO2 gas is used to prepare the lixiviant
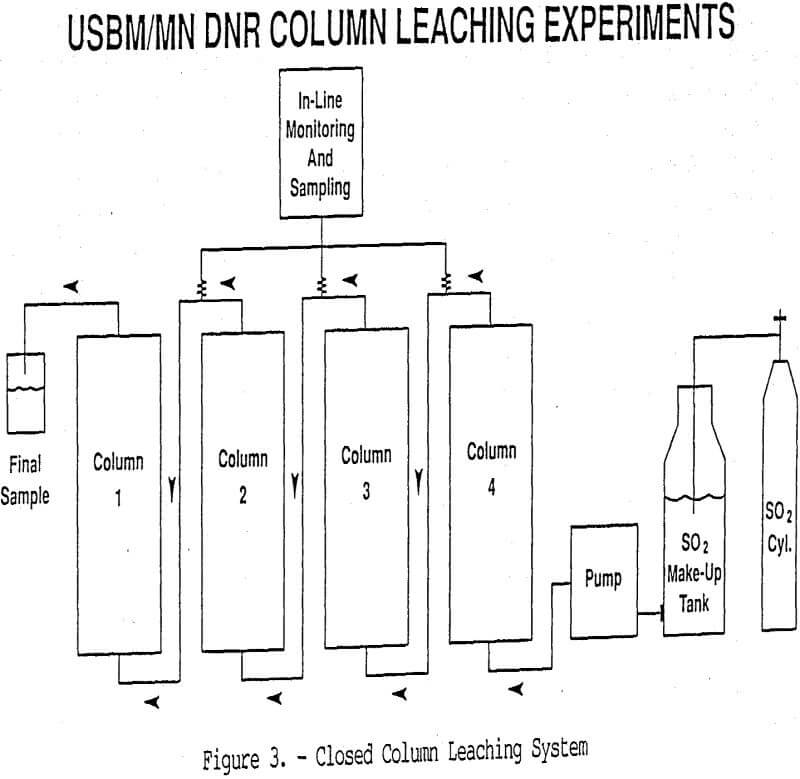
in a continuous batch fashion (Figure 3). This eliminates materials handling problems and allows for a completely closed leaching system to be maintained. A closed collection vessel pressurized with an inert gas (nitrogen, argon) ensures that unreacted SO2 stays in solution. Such a leaching system allows for an assessment of the leaching chemistry as a function of time, including the total amount of SO2 consumed. In line monitoring and computerized data acquisition are also utilized to obtain real-time data on pH, Eh, temperature, and pressure.
Leaching experiments conducted on samples of drill core are also an effective means to evaluate leaching chemistry and yield data not obtained from column leaching tests. In addition to providing chemical data, core leaching experiments will also provide information on permeability changes resulting from mineral dissolution, precipitation, or clay hydration. This is important since the maintenance of adequate permeability is critical to the success of any in situ leach mining operation. In a situation where the ore mineralization is fracture-hosted, core leaching experiments may yield significantly different data than column tests due to increased ore mineral accessibility (Paulson, 1989). A variety of configurations and materials can be used to conduct core leaching tests (Paulson and Kuhlman, 1989), but the basic design is similar in all: a section of core is placed within a piece of pipe, encased in epoxy and sealed with end caps. Lixiviant is pumped through the core in an axial direction and collected in a closed vessel prior to sampling and analysis (Paulson and Kuhlman, 1989). The differential pressure across the sample is monitored using a pressure transducer in order to evaluate real-time permeability changes. Upon completion of the experiment, the remaining core material is impregnated with epoxy and prepared for petrologic studies.
In summary, the combination pre- and post-leach petrologic studies with a variety of leaching experiments are intended to yield information which will ultimately allow for predictions as to what can be expected when applying in situ leach mining to an actual Mn deposit. Site-specific information is also a vital part of this assessment. And while it is not possible to attempt to scale a deposit down to a laboratory test, such tests certainly provide valuable and necessary data to evaluate a deposit’s suitability for in situ leach mining.
Geologic Characterization
Prior to conducting any type of leaching experiments, it is important to have a thorough understanding of the nature of the material to be leached, as this will be essential for the interpretation of the leaching data and the correlation of the leaching characteristics exhibited by each ore type. It is particularly important to characterize the relationships between the various Mn and gangue minerals in the rock and the main fluid flow channels, as this is where most of the reactions will occur. This includes a detailed study of the chemistry and reactivity of the minerals located along these channels.
A number of different techniques are utilized for the characterization including, transmitted and reflected light microscopy, X-ray diffractometry, scanning electron microscopy, and electron probe microanalysis. The porosity and permeability of the samples is also measured to document changes in these properties as a result of mineral dissolution and precipitation reactions. A very important aspect of the characterization is the necessity for the pre- leach samples to be representative of the actual samples being leached. The failure to meet this requirement can make the correlation of pre- and post-leach characterization quite difficult. The acquisition of representative samples is often complicated by the significant variability in ore mineralogy and grade over a relatively short distance. Selection of representative samples is extremely difficult without the use of characterization techniques. These techniques provide the basis upon which a comparison can be made and “representativeness” can be judged.
Sample preparation is an important part of the characterization work, particularly when the samples are friable and susceptible to alteration during cutting, grinding, and polishing operations. Post-leach samples are often difficult to work with due to dissolution of the Mn minerals which comprise a significant fraction of the total rock volume. Thus, special preparation techniques are utilized which maintain the textural and mineralogical features to be studied. These techniques include the use of a variety of epoxies and impregnation procedures (Brink et al., 1990).
Transmitted and reflected light microscopy are used to conduct the initial characterization of the samples and to identify areas for more detailed study using scanning electron microscopy and electron probe microanalyses. Among the readily-observable features examined are (1) the associations of ore and gangue minerals to microfractures; (2) the frequency, length, width, and interconnectivity of fractures; and (3) the grain sizes, boundary relationships, and effective surface areas of both ore and gangue minerals (Brink et al., 1990). In addition to visual observations, systematic point counting techniques are also employed to quantify the modal mineralogy of the sample. This information is combined with the chemical compositions of the reactive minerals to predict Mn recovery during in situ leach mining.
After thorough examination by optical microscopy, the scanning electron microscope (SEM) and electron microprobe are used to examine and analyze particular areas of the samples at high magnification. The SEM can be used to obtain information on surface morphology and qualitative chemical data. A particularly useful feature of the SEM is the capability to map specific elements within the samples and also to overlay these maps as a way to illustrate the distribution of particular minerals in the sample. The SEM is also useful for determining the width and frequency of microfractures which may act as primary or secondary flow channels during in situ mining.
The electron microprobe is used to determine the qualitative or quantitative chemical composition of an area as small as 1 micron. This instrument is particularly useful to assess the effects of the lixiviant on particular minerals in the system. For example, pre- and post-leach samples are compared to determine the extent of metals removal as a result of leaching with SO2. This not only yields information on Mn removal efficiency, but it also helps to determine which gangue minerals consume SO2 and to what extent. Traverses across mineral grains can identify zoning and can also yield information useful for evaluating leaching kinetics.
X-ray diffractometry (XRD) is another method used to obtain mineralogical data. The bulk mineralogy of an unidentified material can be determined relatively quickly using XRD, as each mineral has a distinctive X-ray pattern. X-ray diffractometry is particularly useful for analyzing very fine grained minerals, such as clay minerals, which are often difficult to identify using optical microscopy. The clay mineralogy of a sample can be very important, as ion exchange and hydration reactions can have significant effects on solution chemistry and rock permeability, respectively.
Deposit Characterization
In addition to geologic characterization studies performed in the laboratory, geologic field data is also crucial to evaluating a deposit for its amenability to in situ mining. Included in the field evaluation are (1) determinations of parameters such as the orebody geometry; (2) structural geology, including fracture and fault orientations; (3) porosity and permeability of both the ore and host rock; and 4) regional and local hydrologic conditions. These parameters are evaluated using a variety of methods including core and cuttings from drilling activity; downhole geophysical logging techniques such as gamma, neutron, sonic, resistivity, and temperature logs; surface electromagnetic and seismic techniques; borehole cameras; and downhole hydrologic techniques such as spinner surveys, packer tests, pumping tests, and injection tests. In addition, information generated from previous exploration or geological surveys is utilized for the deposit evaluation. This includes geologic maps, cross-sections, and drill core and cuttings.
Mn Recovery
In addition to favorable leaching characteristics, a viable metal recovery process is also needed to develop a complete in situ leach mining system. One without the other is an incomplete system. A preliminary assessment of potential Mn recovery options first required the identification of saleable end products. Due to current market conditions, Mn specialty products such as battery grade manganese dioxide and Mn chemicals appear to be the most likely target end products at this point (Redden, 1990). Since no viable Mn recovery process currently exists, research is being conducted to identify feasible recovery schemes that fit into the overall in situ leach mining design. A Mn recovery process may require initial steps such as solvent extraction or ion exchange to purify and concentrate the Mn prior to recovery. Recovery methods being investigated include precipitation (Hepworth, 1989), crystallization, and electrolytic techniques (Redden, 1990). After technically-feasible schemes are identified and refined, evaluations will be performed to determine the economic feasibility.
Prior to any field test projects, an in situ leach mining cost model will be developed to evaluate the economics of the whole mining process. This cost model will include parameters such as reagent costs, well field development and well completion costs, ore grade, expected recovery, surface processing costs, environmental monitoring costs, ore zone permeability, and a break-even selling price for the end product.
Results/Status of Bureau of Mines Research
The Bureau of Mines is using the systematic geologic and geochemical characterization approach described in section II to evaluate the potential for recovering manganese from domestic reserves by in situ leach mining. To show how this approach has yielded new information on the feasibility of in situ leaching of manganese, preliminary data from the Bureau’s research program will be presented. Experimental work includes column, core, and batch leaching tests. Geologic characterization of ore samples before and after leaching has been completed to determine primary reaction mechanisms and leaching efficiency. Optimum solution chemistry conditions for selective leaching (i.e. pH and lixiviant strength) have been investigated experimentally by determining leaching reaction kinetics for various conditions. Field evaluation of manganese deposits has been initiated by core drilling and preparing field sites to conduct downhole geophysical and hydrologic tests. Recent advances in each of these areas will be discussed in detail in the following sections.
Leaching Experiments
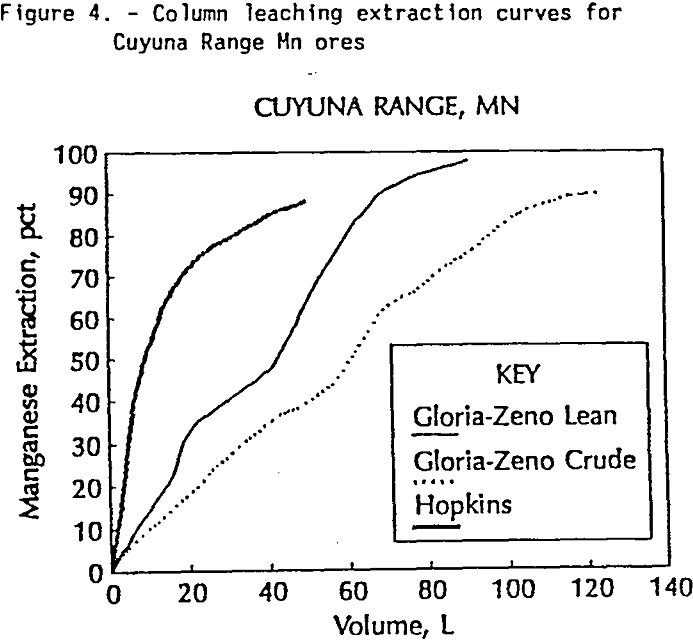
Column Tests: Twelve column leaching tests were conducted on manganese ores from three domestic deposits that had previously shown good leaching characteristics in SO2 solution (Pahlman and Khalafalla, 1988). These included ores from Artillery Peak in Arizona, the Pioche District in Nevada, and the Cuyuna Range in Minnesota. The leach
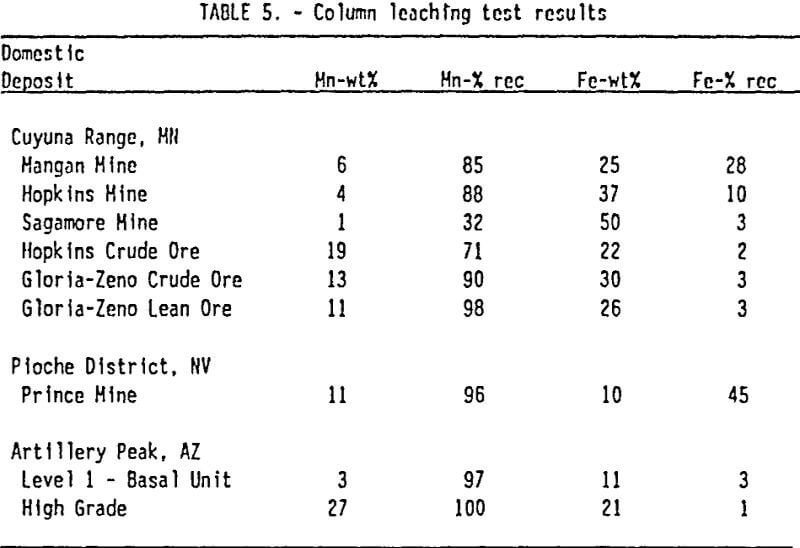
tests followed the experimental design described by Pahlman and Khalafalla (1988). Tests were conducted in 10-cm. diameter columns that contained 3500 gm of -1 +½ inch pieces of crushed ore. Solutions of 5- volume-%-SO2 were dripped on top of the column at a rate of 1 mL/min. The column was not tightly sealed and remained unsaturated throughout the experiment. Tests were run until the manganese in the leachant from the column dropped below 1 g/l. The duration of the tests ranged between 17-85 days.
Results from the column leach tests are displayed in Table 5. The data indicate that manganese can be recovered from all the ores tested by leaching with SO2 solutions. Results also show that manganese is selectively leached with respect to iron in all the ores tested. Extraction of manganese ranged from 30 to 98 percent. Extraction of iron ranged from 2 to 40 percent. The slope of calculated extraction curves as shown in Figure 4 may reflect differences in the fundamental leaching properties of the ores being tested. For example, the extraction curves for the Gloria-Zeno Mine and the Hopkins Mine both show high overall recovery of manganese, but there is a change in slope of the curve for the Gloria-Zeno ore which is not observed in the Hopkins ore. Although the manganese mineralogy is similar for both ores there is a bimodal textural occurrence of manganese in the Gloria-Zeno ore which contributes to the two stage leaching pattern observed in the column tests.
After completion of preliminary column leach tests, ores from the Gloria-Zeno mine were selected for detailed study in a closed system column leach test. The column leach experimental design was modified to answer several questions that were raised after the first series of column leach tests. In the closed column design, information on the ratio of consumption of lixiviant to production of manganese could be answered because sulfur dioxide can not leave the system. The closed column tests are conducted under saturated conditions which will more closely represent conditions during in situ mining. On-line measurements of pH, conductivity, and temperature have been incorporated into the design of the new column leaching tests. Variation in pH had previously been documented during batch leach tests of manganese oxide, but this change was not observed in the first series of unsaturated column leach tests. In the closed column tests, on-line measurements have verified that there is a significant change in pH with leaching, which may have important implications on the overall leaching efficiency and for development of compatible metallurgical recovery techniques. Finally, fluids
recovered from the closed columns tests on Gloria- Zeno ores had much higher maximum manganese concentrations (26 g/L) than the open system tests (5 to 11 g/L). This difference is most likely due to longer solution residence times and minimal SO2 loss in the closed system experiment.
Batch Tests: Batch leaching tests of manganese ore were designed to complement the information from the column tests. One type of batch leach test consisted of placing a cube-shaped sample in an inert plastic sample basket suspended in a stirred, stoppered Erlenmeyer flask containing an aqueous SO2 solution. Small samples of the solution were removed periodically with a filtered syringe for chemical analysis. An equivalent amount of fresh lixiviant was added to maintain a constant fluid/rock ratio. Solution pH, Eh, and temperature were measured by probes inserted through the stopper.
The cubic samples of ore were approximately one cm on a side, cut with faces parallel and perpendicular to bedding. The cubes were photographed, weighed and measured before leaching. Petrographic thin sections and briquettes for optical and electron microscopy were prepared from the rock sample immediately adjacent to the cube faces, and examined as ‘pre-leach’ sample. After a sample was leached; it was removed, dried, weighed, photographed, and again examined by light and electron microscopy. In examining the sample, special attention was paid to textural changes resulting from lixiviant/mineral interactions. The batch tests of ores from the Cuyuna Range and Emily District in northern Minnesota, confirmed the results from column tests of high manganese recovery (75-100%) and high selectivity for manganese relative to iron [i.e. Mn/(Mn+Fe) in solution >0.975]. However, observed leaching rates of ores with different manganese mineralogies (manganite versus pyrolusite) did not agree with predictions based on earlier batch leaching tests of single minerals (Pahlman and Khalafalla, 1988). These earlier tests and a theoretical evaluation of reaction equilibria (see the section on selective oxide dissolution) indicate that pyrolusite should leach more readily than manganite in SO2 solution. Recent batch leaching tests show the reverse: the manganite ore samples leach more readily than the pyrolusite samples.
This behavior can be explained by consideration of the ore mineral textures identified in each sample. The Emily manganite sample was composed of massive manganite with inclusions of hematite and some quartz. As leaching progressed, non-reactive hematite and quartz spalled off the reacting surface, exposing fresh manganite to the lixiviant. As the experiment proceeded to completion, all the manganite dissolved, leaving a slurry of insoluble gangue residue on the bottom of the beaker. The Gloria-Zeno pyrolusite ore sample, however, occurs in bedding planes and fracture fillings surrounded by and intimately associated with quartz and hematite. In order to remove the manganese from that sample, the lixiviant must penetrate the rock sample, the manganese dissolution reactions must occur, and the dissolved manganese must exit the sample. Thus, the ore texture of the Gloria-Zeno sample provided a kinetic barrier to leaching not present in the Emily ores. This illustrates the significant control of texture on leaching characteristics and the need for detailed geologic characterization studies to interpret such leaching characteristics.
Core Leach Tests: One core leaching test was completed on a high grade sample from the Gloria-Zeno mine in Minnesota. The concentration of manganese in the leachant reached a maximum of approximately 40 g/L while iron concentration was near 0.5 g/L. Data also showed there is a change in pH from an input level of about 0.7 to an output of about 3.5.
The core leach test results demonstrate the complexity of the variables controlling the leaching process. Permeability changes in the sample were observed during leaching. Initially, distilled deionized water was pumped through the sample. However, when aqueous SO2 injection was started, the permeability rapidly decreased causing the system pressure to reach the maximum allowable level. At that point, the core was inverted and pumping was initiated from the opposite end of the core. In a field setting, this procedure might be analogous to switching a recovery well to an injection well. Injecting in the opposite direction resulted in a significant increase in permeability. Permeability changes were continuous and variable during the operation of this experiment. One possible explanation for the observed permeability variation is that the dissolution of manganese, and consequent erosion of solid iron minerals would plug up flow channels as reactions proceeded. This would act to force lixiviant into new flow channels and open up new areas of fresh manganese mineralization. With time, there should be an increase in overall permeability by the selective removal of manganese minerals. Preliminary geologic characterization analyses support these hypotheses.
Geologic Characterization
Post-leach ores from the batch, column, and core leaching tests were investigated by techniques described above in the experimental approach section. Examination of mineralogic and lithologic differences between ore samples can answer several questions about the differences in extraction ratios achieved in leaching tests. For example the lowest recovery of manganese achieved (30 wt%) was from an ore sample with a very low initial manganese content similar to country rock surrounding a deposit. Since the total volume of manganese minerals was low, the manganese in this ore was not easily accessible to leaching solutions, new flow paths to manganese never developed, and good recovery was not achieved. One of the highest manganese recoveries (97 wt%) was recovered within 15 days from a very porous sandstone which facilitates rapid and complete contact of lixiviant with ore minerals. The highest amount of manganese recovered (98 wt%) was extracted In 80 days time from the Gloria-Zeno ore which has a bimodal occurrence of manganese mineralization. Pyrolusite occurs in fracture fillings and disseminated in the matrix of this ore. With sufficient time, both types of manganese were removed from the ore.
Another major influence on the leachability of ores is the physical relationship between the ore and gangue minerals. For example, theory indicates that the mineral pyrolusite should be more leachable than the mineral manganite in sulfur dioxide solution. However, Bureau experiments on ore samples containing these minerals, showed that the opposite occurred: manganite was more leachable than pyrolusite. Comparison of pre- and post- leach samples using optical and electron microscopy show that the pyrolusite occurs in the rock surrounded by gangue minerals, while the occurrence of manganite in fractures allows the lixiviant more access for leaching. This example highlights the importance of geologic characterization in applying leaching to individual deposits.
Microscopic examination of ores leached by sulfurous acid also offer insight into the chemical reactions occurring during dissolution. In post- leach examination of massive manganite ores which contain hematite inclusions, sulfurous acid dissolves the host manganite while leaving the hematite unaffected. In pyrolusite-hematite-quartz ore a similar process is observed in which a residue of hematite and quartz was left unleached. In the case of a batch leach test of cryptomelane ore from the Emily District, the suspension of hematite and quartz was left in the flask after all the cryptomelane had been dissolved. These observations of the physical results of leaching supports the selectivity of sulfurous acid for manganese oxides over certain iron oxides, a result that is also indicated in the leach chemistry, and changing permeability conditions.
Deposit Characterization
In addition to mineral texture and leaching tests, the specific geology of a deposit on a regional scale is an important step in assessing an area’s potential for in situ leach mining. Because of the significance of the size of the Cuyuna Range in Minnesota to total domestic manganese reserves, field studies of this area were initiated to begin the Bureau’s field characterization program.
Iron formation in the Cuyuna Range of Minnesota contains low grade, high tonnage deposits of manganese oxides and manganese carbonate. Core drilling was completed at the Gloria-Zeno mine site to a total depth of 1200 feet and at the Emily District to a total depth of 800 feet. Core was successfully recovered from the ore horizon and from the hanging and footwall formations. Preliminary assessment of the geology indicates that the zone of oxidized manganese mineralization at the Gloria-Zeno Mine extends much deeper than was previously thought and that previous structural interpretations of the site will require some modification. Because the footwall formations of the Cuyuna Range have not been well characterized, data gained from this drill hole will for the first time allow accurate determination of the permeability of the leach zone footwall. The holes were not subjected to final abandonment procedures so that future down hole geophysical and hydrologic testing of the deposits can be conducted after thorough characterization of the ore zone.
Conclusions
The Bureau has developed a research protocol for investigating how advanced leach mining techniques could be applied to critical and strategic mineral commodities. This research protocol has been used to investigate the mining of manganese oxide ores by in situ leaching. Many of the techniques found to be significant research tools when developing in situ mining for base metals were used to define leaching parameters for manganese. These techniques include geologic characterization by optical microscopy, electron microprobe analysis, and x-ray diffraction analysis; geochemical characterization by batch, column, and core leaching tests; and field characterization by core drilling, geophysical, and hydrologic tests.
Results showed that efficient leaching of manganese oxide mineral deposits requires a lixiviant that is chemically selective for manganese because of the intimate relationship between iron and manganese oxide minerals in the deposits studied. New metallurgical recovery processes are needed to recover saleable products of manganese from manganese sulfate solutions in a way that allows solution recycling. Stratified, steeply dipping ore zones typical of some manganese deposits will require development of well patterns designed to utilize stratigraphy to control solution flow. The Bureau has used proven techniques and incorporated commodity specific research to develop a process oriented approach to the in situ leaching of manganese.
Acknowledgment
The authors wish to thank Lorin Redden from the Bureau of Mines Salt Lake Research Center for his input pertaining to Mn recovery research.
