A great deal has been written of late regarding the loss of silver in assaying; very discordant results have been published by different writers, and much uncertainty exists concerning even approximate losses in a careful determination of silver, as represented by a fire-assay.
In the present paper it has been the aim of the writer to determine as accurately as possible the losses sustained by silver, under certain specified conditions, during the process of cupellation. Such losses are well known to be due to absorption by the cupel and volatilization. The conditions, however, which govern these losses are not believed by the writer to be so well understood. The most important of these are:
- The effect of variable quantities of silver used.
- The temperature of cupellation.
- The character and quantity of impurities in the lead button.
- The weight of the lead button.
- The nature of the cupel.
The following experiments were made for the purpose of demonstrating, as nearly as possible, the total loss of silver in cupellation under the most favorable conditions to be obtained in commercial assaying.
Chemically pure silver and pure lead were taken. The latter was obtained from test-lead, first melted into a bar, from which pieces of the desired weight were cut and accurately weighed.
The experiments recorded in this paper were planned by the writer, but executed throughout by Mr. J. N. Walker, whose ability as an accurate, careful and painstaking assayer is too well known and recognized throughout the West to require any further endorsement from me.
The conditions governing the loss of silver in cupellation were examined as carefully and completely as was thought possible, with the exception of the comparative effect of impurities in the lead button, which will be the subject of a future paper.
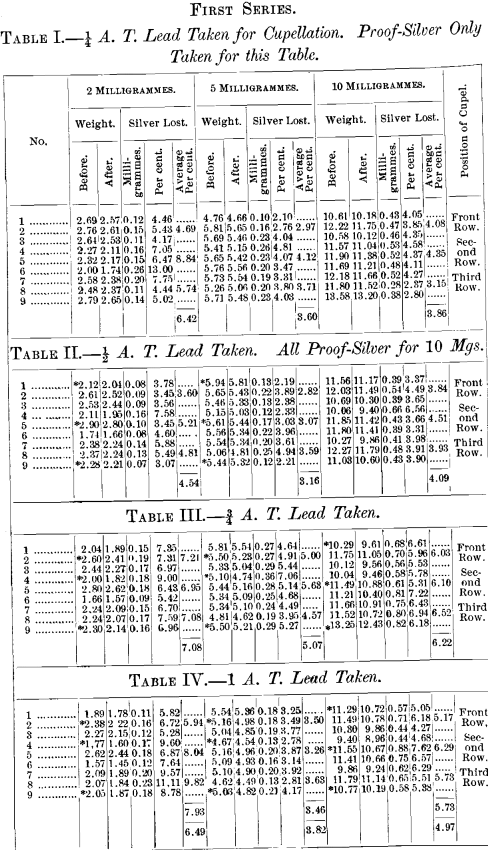
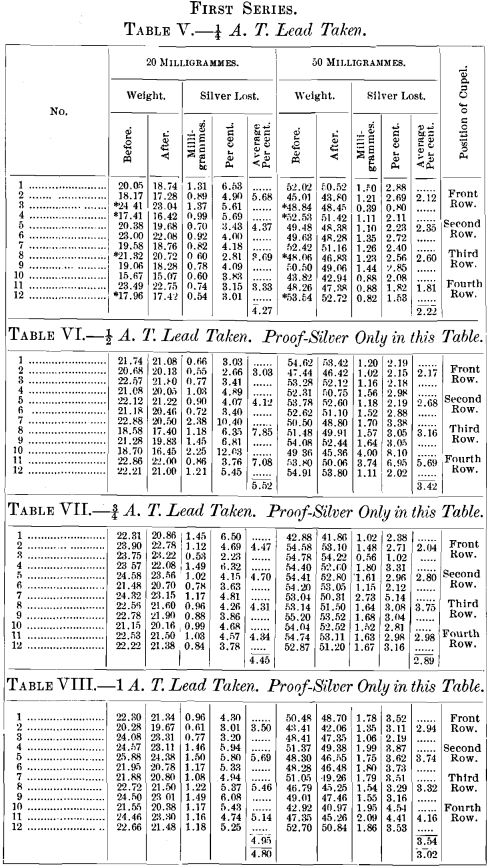
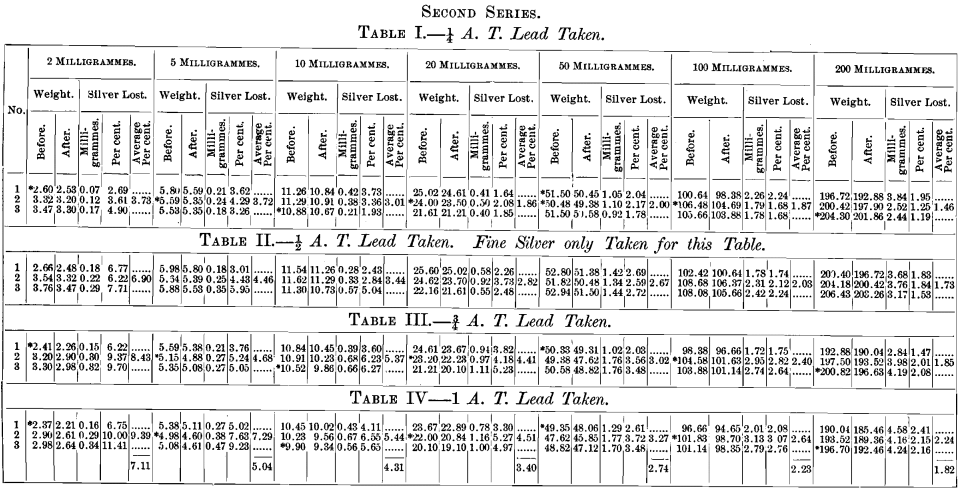
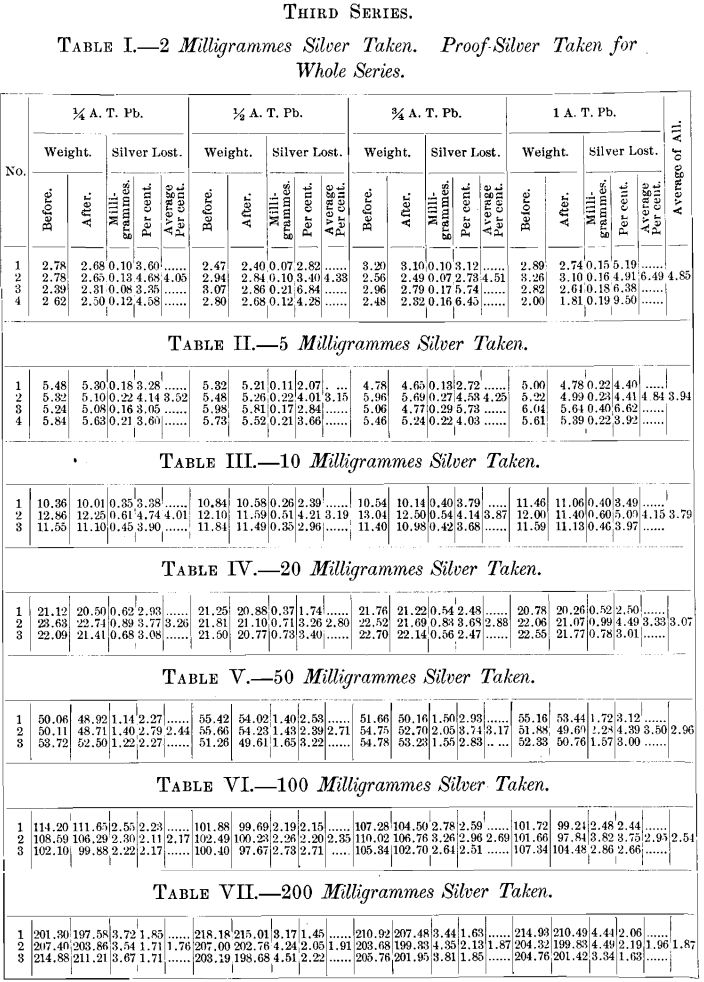
A brief outline of the plan of the experiments is as follows: All assays were made in triplicate. The effect of variable quantities of silver used was determined by taking carefully weighed quantities representing approximately 2, 5, 10, 20, 50, 100 and 200 milligrammes. The effect of temperature was determined by cupelling not less than three rows in any experiment, whilst, in several, four rows were taken. Coolers for the back-row were invariably used, and the greatest possible care was taken to preserve a uniform and proper heat throughout the operation.

The effect and character of impurities in the lead button, as before stated, will not be made a part of this paper. The effect of a variable quantity of lead used was determined by taking a quarter, a half, three-quarters and a whole assay-ton for each series of experiments.
The influence of the nature of the cupel will be treated in this paper only so far as to demonstrate the reliability of the cupels used by recording the results of a series of experiments obtained by using, in addition to the cupels of our own make, two other kinds as standards. The latter were obtained from the New York Assay Office and the San Francisco Mint, through the kindness of Mr. Andrew Mason, the Superintendent of the New York Assay Office, and Mr. Ben W. Day, of the San Francisco Mint.
Three series of experiments were made to examine the effect of the variable conditions, as above stated, from every conceivable standpoint, as well as to check the results obtained.
The first series consisted in cupelling at one time not more than two different quantities of silver with a uniform weight of lead. The position of the cupels in this series was as follows:
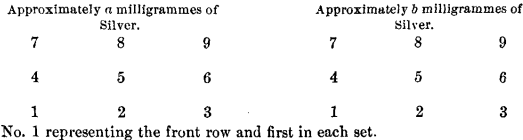
In the experiments with 20 and 50 milligrammes silver four rows were cupelled at one time instead of three as shown above, otherwise the positions were the same.
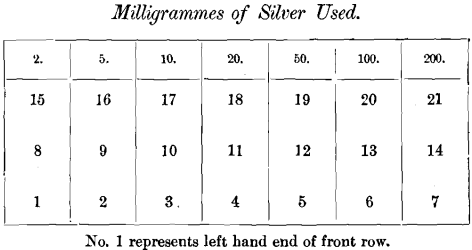
In the second series the same weight of lead was again used, but seven different weights of silver were taken for each cupellation. The position of the cupels was as follows:
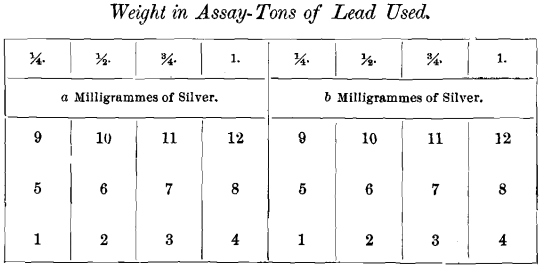
In the third series not more than two different weights of silver, but four different weights of lead, were used in each cupellation. The position of the cupels was as follows:
In the final series of experiments, where the comparative absorption of the different cupels was determined, the same quantity of silver, and not more than two different weights of lead, were used in each cupellation.
The position of the cupels was as follows :
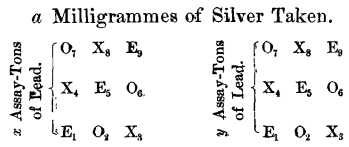
No. 1 represents first button in front row of each set.
Nos. 1, 5 and 9 represent Everett cupels (E).
Nos. 2, 6 and 7 represent N. Y. Assay Office cupels (O).
Nos. 3, 4 and 8 represent S. F. Mint cupels (X).
The results of the above series of cupellations are given in the tables appended to this paper. The buttons represented by each table were cupelled at one and the same time, excepting Tables I., II., III. and IV., in which the exceptions are the 10-mg. buttons, which were cupelled by themselves; those of Tables I. and II. in one operation, and those of Tables III. and TV. in another.
Throughout the whole of the above series, wherever it is not distinctly stated that proof-silver only was used, silver buttons were used which had been obtained from a previous cupellation, the exceptions being those marked by an asterisk in the tables.
The object in doing this was to determine if any lead had been left in the button, which would be shown by the check, containing only proof-silver, used in each case.
Throughout the foregoing experiments discrepancies in results are frequent; but it is believed by the writer that a repetition of the experiments made in the most careful manner would not show any smaller number, with one possible exception. It was discovered while conducting the above experiments that, where buttons of unequal size were cupelled at the same time, it was of the greatest importance to lower the temperature of the muffle as soon as the smaller buttons had “ uncovered.”
In some cases, where buttons of different sizes were all put in the muffle at the same time, and the heat was rather low for the beginning, the small buttons would “ uncover ” and perhaps be nearly half cupelled before the large ones started and the temperature could be lowered to the proper degree. One way to overcome this would be to put in the large buttons first. This part of the operation is believed by the writer to be very important, as the condition is a common one where many scorification-assays are made.
In all the above experiments, the cupellations were made at a temperature as nearly uniform as possible. Dry cupels were invariably used, and were allowed to remain in the muffle until of the same temperature, before the buttons were put in.
The cupellation was started by closing the front of the muffle and allowing it to remain closed until all buttons were bright, or “ uncovered,” when the temperature would be lowered as quickly as possible, and the cupellation would then be conducted at a low heat until the end. The cupels, however, were not pushed back just before the buttons “ brightened,” nor afterwards, a condition stated as necessary in most text-books on assaying, but not found so by the writer when using pure lead and pure silver, and in quantities as above.
In some cases, where the temperature was quite low at the end, the front of the muffle would be closed just before “brightening,” and left closed for several minutes, to insure the removal of all the lead.
No distinction is made in this paper between the loss by absorption and volatilization. The writer expects to continue experiments along this line, and in a future paper to discuss this matter more fully.