Table of Contents
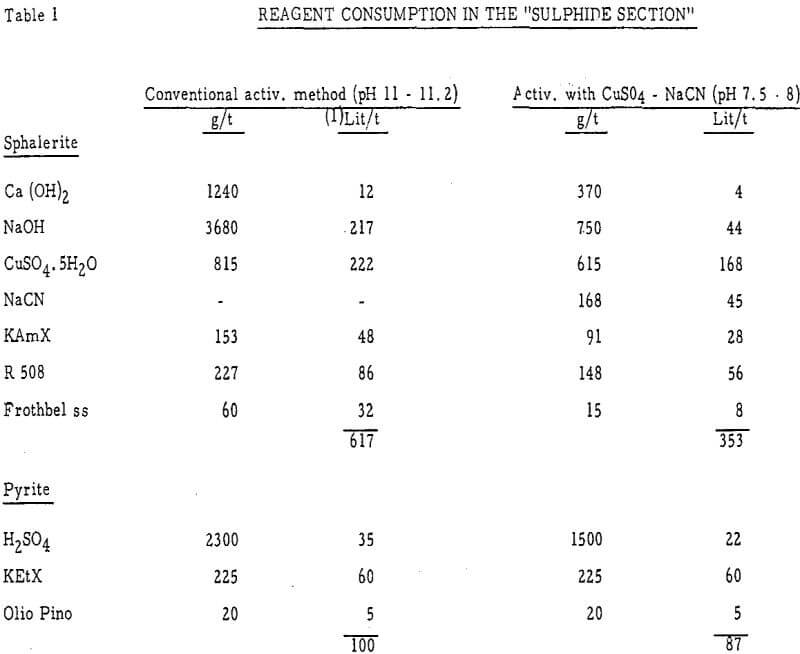
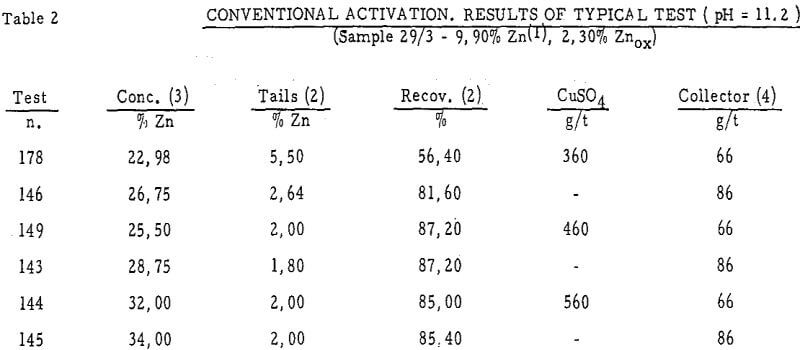
The main constituent of the feed to the “Sulphide Section” (31 t/h), in the F. Sartori Plant (6000 t/d) of the Miniere di Monteponi(Sardinia), is the ore of the Campo Pisano – Funtanaperda Mine. Difficulties have always been experienced in the treatment of this ore, owing to the extremely fine Pyrite-Sphalerite intergrowth and to the high Pyrite and soluble salts contents.
Preliminary Considerations
In connection with what has been reported above, the depressant, needed for pyrite, should:
- Work at neutral pH
- Not be consumed by the soluble salts
- Be most effective in the same conditions, in which the soluble salts are less deleterious or in which some of them may even enhance the action of the depressant.
It is known that ferrous and ferric ions, in presence of copper sulphate, and of xanthate as a collector, tend to lower the floatability of pyrite with respect to that of the other sulphides.
When working with pure solutions containing CuSO4 and at least 0.03 g/l of Fe as ferrous sulphate, one may succeed in preventing the pyrite-air bubble contact at pH 6.2 to 9.0, if the surface is slightly oxidized, and at pH 4.5-6.2, if the surface is fresh (see fig. 2 at NaCN = 0).
Sodium cyanide seemed to be an ideal reagent also in order to benefit from the presence of dissolved Fe in the pulp.
Laboratory Tests – Part I
Pure minerals, dry ground to -65 mesh and accurately deslimed, were placed in a 100 cc test tube in sufficient quantity to give a density of 5 to 30% solids with 50 cc of water. Conditioning of activating and depressing agents was generally limited to 1 min; flotation was obtained by agitating the suspension about 10 sec after addition of collector and frother.
The first tests on sphalerite showed the considerable importance of the CuSO4. 5H2O/NaCN ratio, especially at pH about neutral. Fig. 10, obtained at pH = 7.3, indicates that, in the test conditions, the most interesting values of the mentioned ratio are above 3.5, and that values lower than 2.5 may bring about complete lack of floatability; one may remark that the latter ratio corresponds to the stoichiometric quantities of CuSO4. 5H2O and NaCN for the formation of cuprous cyanide (CuCN).
The pyrite stops floating when pH 6 to 7 is reached; at higher pH floatability increases again but very slowly. Curves 4 show that, when copper sulphate is absent, cyanide effectively depresses pyrite only at pH above 9.
Laboratory Tests – Part II
CuCN is not an activator for sphalerite; therefore the latter could be activated in its presence only when excess Cu is also present, either because added initially or because subsequently freed upon formation of HCN.
However, such a hypothesis, simple and therefore attractive, is partially contradicted by fig. 12 (curve 3) and by the series of tests that follows.
Furthermore, it seemed improbable that the selectivity obtained in the laboratory cell was entirely due to the existence of a zone, representing “floatability for sphalerite” and “non floatability for pyrite”
A saturated solution of CuCN (C.P.), having 2.2 mg/l concentration referred to Cu, was used to condition sphalerite at 5% solids in closed cylinder tests, and turned out to be an excellent activator. The lack of a depressing action was attributed to the low concentration, but it was impossible to prove the correctness of this assumption, because a more concentrated solution of CuCN, starting from the solid reagent, could not be prepared.
Several tests were made, starting from copper sulphate and cyanide, with 1 increasing concentrations of the two reagents; these were mixed in the water used for the test about 20min before adding the mineral, and their ratio was varied from 2. 5 to 1.
Depressing Action on “Pre-activated” Sphalerite
In order to establish the relative importance of activation and depression intensities, some c.c. tests were carried out on sphalerite that had been strongly activated (4 min in 10 g/l CuSO4·5H2O) and subsequently washed.
The results were as follows :
a. Neither cyanide alone (up to 52 mg/l), nor the pre-mixed copper sulphate-cyanide combinations (up to 24 mg/l ref. to Cu) in the various ratios so far considered gave any sign of depressing action.
b. When copper sulphate and cyanide solutions were added separately but simultaneously to the water lying above the settled mineral, their depressing action was considerable and increased to a maximum, with the increase of the CuSO4·5H2O/NaCN ratio, as shown in table 7. One may point out that the strongest depression of activated sphalerite occurs with those ratios and methods of reagent additions that favor the presence in the pulp of the “intermediate products”.
In the last paragraph, one could explicitly speak of “depression”, because the sphalerite of the tests had been sufficiently activated to give, without further activating conditioning, what looked like “maximum” floatability. On the contrary, in the earlier tests on non-preactivated minerals, one could not always tell whether the cause of low floatability was depression or insufficient activation. To gain a better understanding of this point and evaluate the activating ability of the different reagent combinations, some activation tests were carried out with very dilute solutions of: CuSO4·5H2O, CuSO4·5H2O+NaCN pre-mixed in different ratios, CuSO4·5H2O+NaCN separately added, CuCN (C. P.). All these solutions activated sphalerite so effectively that the first addition of Collector (4 mg/l) was sufficient to give “maximum” floatability at initial concentrations, referred to Cu, of 1.5×10-¹ mg/l in fresh water, and of 1.5×10 -5 mg/l in distilled water.
In the paragraph “Sphalerite” a stronger depressing action had been reported with the lower copper sulphate-cyanide ratios (pre-mixed reagents) rather than with the higher ones. Low ratios are the most depressing also when, instead of low reagent concentrations, the solution contains a higher percent of solids (sphalerite).
The importance of reagent concentrations, before and during activation, on the behaviour of sphalerite in presence of copper sulphate-cyanide combinations is illustrated by table 9, which takes into special consideration the percentage of reactive or actively adsorbing solids. Table 9 was obtained from tests in which the two reagents were added separately, as in the plant practice.
About NaCN alone, as investigated through its effects on activated and washed sphalerite, one may conclude as follows: also at neutral pH it has a definite depressing action, which strictly depends on the degree of activation and rapidly disappears with the increase of the latter.
Pyrite
The same CuCN saturated solution (2.2 mg/l referred to Cu), already tried on sphalerite, was tested also on pyrite (closed cylinder). A very slight depression was noticed.
As for sphalerite, systematic tests at about neutral pH were carried out with different proportion and concentrations of copper sulphate and cyanide. The pyrite was fresh or pre-treated in CuSO4 solutions.
a. Flotation only. The pre-treatment with CuSO4 has no practical influence on floatability
b. Flotation preceded by conditioning with CuSO4. The higher the Cu concentration the more conspicuous is the depression; the pre-treatment with CuSO4 enhances this effect (6 mg/l) of Cu on pre-treated pyrite have the same depressing action as 12 mg/l on fresh pyrite, see 2 to 4/A, C.
c. Flotation preceded by conditioning with NaCN. Depression increases with NaCN concentration but only to a maximum that is reached at 20-25 mg/l. “Pre-treatment”does not seem to influence floatability.
d. Flotation preceded by conditioning with CuSO4+NaCN separately added. For all copper sulphate-cyanide ratios.
For pyrite the difference between the depressing effect of copper sulphate-cyanide mixtures and that of the two reagents added separately is less pronounced than for sphalerite. On the strength of table 10 and of the considerations already made for sphalerite, one may conclude that conditions, which favour presence in the pulp of cuprous cyanide and “intermediate products”, produce on Pyrite the strongest depressing action; however, cuprocyanide and cyanide seem to affect pyrite more than sphalerite (pH about neutral).
Testing and Application in the Plant
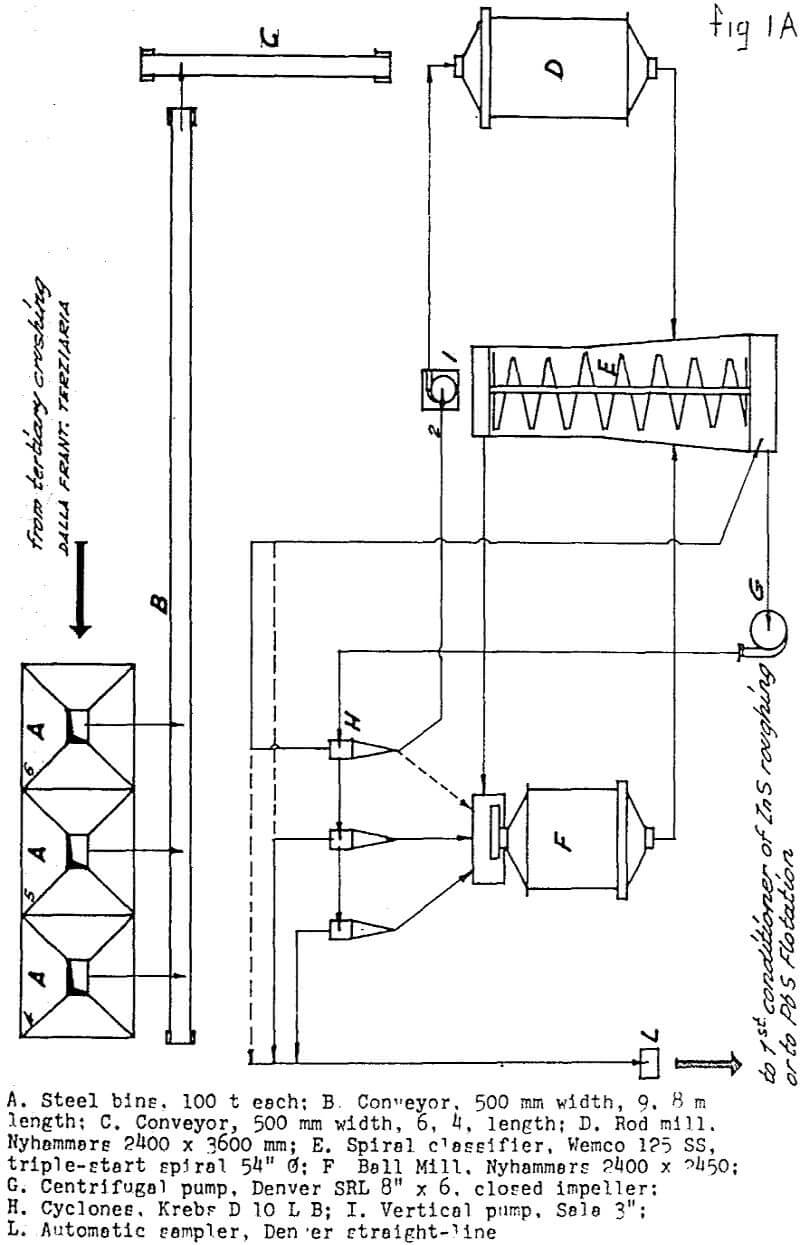
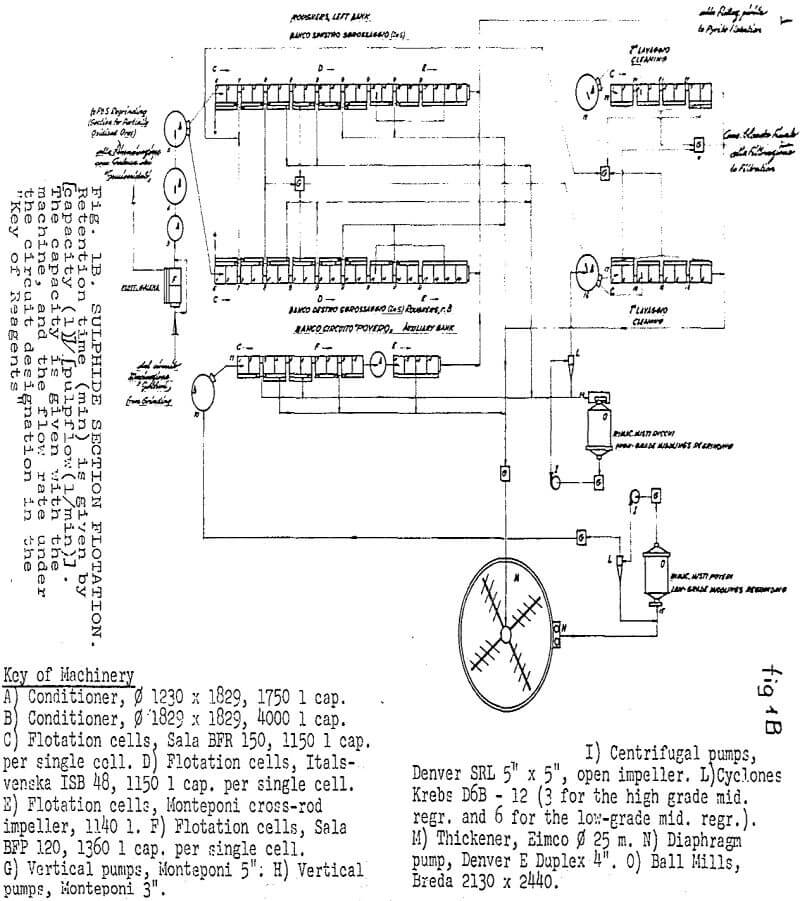
The “Sulphide Section” consists of:
- Grinding in two stages, with cyclones on the 2d stage.
- Two parallel banks of roughers, the rejects of which constitute final tailings.
- One “low-grade middlings” regrinding, which can receive, after thickening, the froth coming from roughing cells 9 to 16, from “auxiliary” cells 3 to 14 (returns), and the rejects from the cleaning circuits. This regrinding operates in closed circuit with cyclones, but classification is based more on quality than on size, and takes place mainly through the froth returning from the “auxiliary bank”
- An “auxiliary bank”, which receives the cyclone overflow of “3”, and the rejects of which constitute final tailings
- One “high-grade middlings” regrinding, which can receive the froth coming from roughing cells 5 to 10 and from “auxiliary” cells 1 to 6.
- First cleaning, which can receive the cyclone overflow of “5” and the froth coming from roughing cells 3 to 6.
- Second cleaning, which can receive the froth from the first cleaning and from roughing cells 1 to 4.
Cyanide and copper sulphate additions were effected in the first conditioner, and collector addition in the third.