Table of Contents
At present, in the United States, municipal solid waste (MSW) is generated at a rate of 3 to 5 lb/d per person. It contains about 8 pct ferrous metallics. Projections indicate -11 MMton/yr of this valuable ferrous scrap is being discarded Discarded MSW magnetics are equivalent to approximately 15 pct of the total U.S. 1982 steel production. It is difficult to recycle this ferrous scrap by direct remelting because the contaminants create problems in the steelmaking furnaces and the steel end product. Copper and Sn are the major metallic contaminants in MSW magnetics that are retained in the steel. They harden and strengthen steels, but also decrease ductility and cause surface hot shortness (cracking in hot-rolled steel products). Lead contamination from the Pb-Sn solder causes problems with furnace refractories. These contaminants force any recycling of MSW magnetics in two possible directions: dilution with blast furnace pig iron or clean scrap in a 10:1 ratio, or removal of the contaminants before remelting.
The detinning industry could treat MSW magnetics to remove the Sn and produce acceptable scrap, but most detinners, using the conventional NaOH leach, prefer not to use this Sn source because of contamination by organic material and Al. Organics absorb Sn-containing leach solution and contaminate the product steel by carryover of the leach solution. Aluminum (in bimetallic cans) consumes the NaOH detinning solution by forming NaAlO2. The formation of NaAlO2 also increases solution viscosity and causes foaming, spillover, and Al contamination of the Sn product.
An additional problem is that many resource recovery plants are not within economical shipping distances of currently operating detinning plants. This problem can probably only be addressed by detinning technology that is workable on a small scale. In response to these problems, the Bureau of Mines has studied nonconventional detinning technology.
A Bureau-funded study by Arthur D. Little, Inc. reviewed existing technology and examined new concepts. It concluded that—
- Conventional detinning technology is not adequate (for the reasons discussed above) to process MSW magnetics on a small scale.
- It is unlikely that nonupgraded MSW magnetics can be detinned economically.
- If MSW magnetic scrap is upgraded to reduce the nonmetallics to reasonably low levels (-1.5 pct), it may be detinned on a small scale economically by a number of processes, including aqueous chemical oxidation and electrolysis.
Stannous fluoborate-fluoboric acid, the solution used to make electrolytic tin-plate, can also be used for electrolytic Sn stripping. A study of this process and its effectiveness in detinning MSW magnetics was initiated. This electrolytic process can be operated at room temperature in a wide range of Sn and fluoboric acid concentrations and between current densities of 20 and 120 A/ft². It was chosen to avoid the high temperatures and the contamination problems of the NaOH leach. Optimum Sn concentration and current density were determined, as were the effects of impurities present in MSW magnetics. Testing began with pure Sn, and then proceeded to postconsumer scrap (PCS) and finally to MSW magnetics. In testing with MSW magnetics, attempts were made to remove lacquer coatings, as suggested by another study.
The Sn recovered from any detinning operation replaces a portion of import requirements. The United States currently imports about 80 pct of its Sn, and the estimated potential recovery from processing about 11 MMton/yr of MSW magnetics is about 30,000 ton/yr, or about half the U.S. consumption.
Cell Design and Experimental Procedure
The material to be detinned was divided equally and placed in two stationary stainless steel wire mesh baskets that served as anodes. Two baskets were used to obtain a uniform Sn deposit on the entire cathode, which consisted of a solid piece of stainless steel with a known exposed area for determining cathode current density. The two wire baskets were 1 in (2.5 cm) apart, and the cathode was between them. The total electrolyte volume was 0.53 gal (2 L), and the solution was circulated with a stainless steel stirring rod. The cell’s potential varied between 1 and 3 V depending on the type of material tested. The cell operated at room temperature. In initial testing, the material used was pure Sn, but later tests used PCS and MSW magnetic scrap. Tests were conducted for the time necessary for complete Sn removal as calculated by—
Tmin = (60) (Xg) (Ccs)/(I) (2.21409)………………………………………………..(1)
where Tmin = stripping time (min),
Xg = weight scrap (g),
Ccs = Sn content of scrap expressed as g Sn/g metal,
I = current (A),
2.21409 = electrochemical equivalent for Sn in the +2 valence state (g/A·h),
and 60 = conversion h to min (min/h).
For example, for a PCS sample
Tmin = (60 min/h) (187.4 g) (0.003 g/g)/(1.5 A) (2.21409 g/A·h) = 10.16 min.
The final Sn content of the scrap was computed by subtracting the amount of pure Sn deposited from the amount of Sn initially present in the scrap and correcting for the Sn concentration in the electrolyte.
Electrolyte Experiments
Determining Electrolyte Composition
Electrolytic removal of Sn using an aqueous stannous fluoborate-fluoboric acid electrolyte was evaluated using operating conditions similar to those used to plate Sn on steel. The cell uses a pure Sn anode. The variables studied were cathode current efficiency, current density, and electrolyte composition.
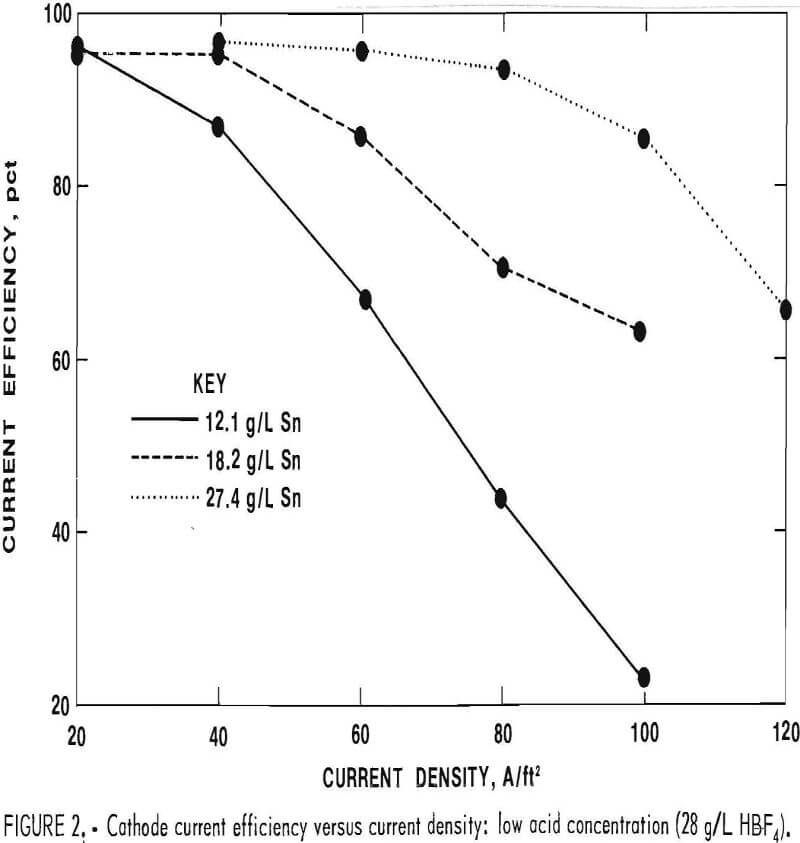
The tested fluoboric acid concentration In the electrolyte was varied between 28 and 174 g/L, and the Sn concentration was varied between 12.1 and 40.0 g/L. Good Sn deposits were obtained (cathode current efficiency >90 pct and minimal amounts of sponge and powder) when Sn
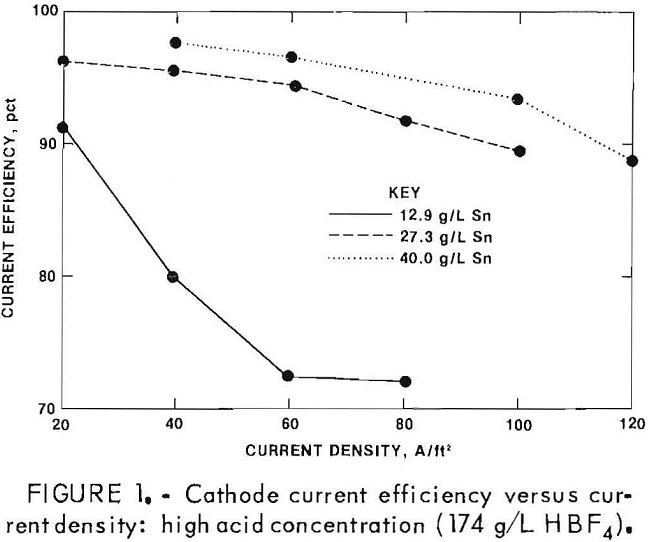
concentration was greater than 25 g/L with current densities at or below 60 A/ft². Gassing occurred at the higher current densities and low Sn levels. This is reflected in lower cathode current efficiencies (figs. 1-2). The acid concentration had little effect on cathode current efficiency; at all acid levels tested, cathode current efficiency was above 90 pct when the Sn concentration and the current density were in acceptable ranges. Tests were also conducted using gelatin and beta naphthol additions to the electrolyte; these additives are used commercially to obtain good throwing power and good tinplate appearance. Based on these results, the following electrolyte composition was chosen:

Since acid concentration had little effect on cathode current efficiency, a moderately low acid concentration was selected from the concentrations used in commercial Sn plating. The gelatin and beta naphthol concentrations used are also representative of commercial practice.
Effects of Impurities in the Electrolyte
Impurities were investigated to determine their effect on cathode current efficiency and the quality of the Sn deposit. The cell used a pure Sn anode. The impurities Al, Cu, and Fe were dissolved into the electrolyte using the elemental metal or oxide; Pb was dissolved as PbCO3. The anode current efficiency was assumed to be 100 pct because the electrolyte’s Sn concentration increased. Any change in cathode current efficiency was caused by the impurity. The effect of impurities on current efficiency at 60 A/ft² is summarized in table 1.
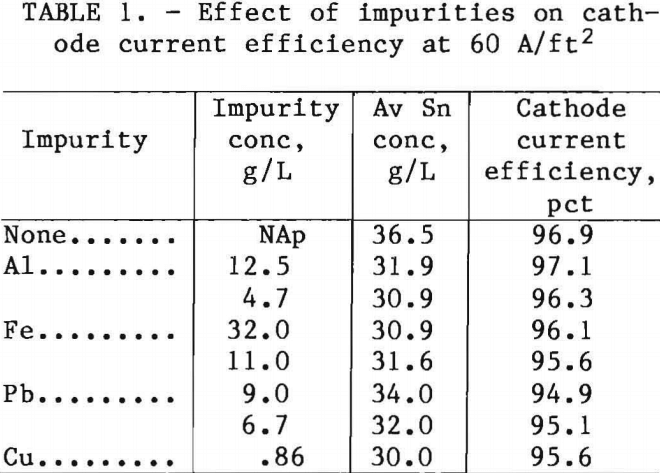
Copper and Pb codeposited with Sn to a much greater degree than Al and Fe. The impurity levels were, in weight percent Cu, up to 3; Pb, up to 10; Al, <0.02; and Fe, <0.40. These impurities had little if any effect on the cathode current efficiency at the concentrations tested. Aluminum in concentrations above 12.5 g/L forms a sludge containing AlF3 and AlF3·3H2O. The concentration of Al in the electrolyte is therefore self-limiting.
Detinning Test Program
Results of Detinning Postconsumer Scrap
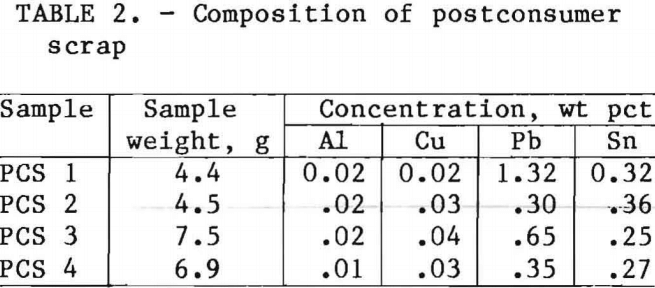
PCS was shredded in a hammer mill with ½-in (1.27-cm) grates. The shredded PCS contained many balled pieces resulting from too long a residence time in the milling zone before passing through the grates. A shearer-type shredder would produce a better sample for detinning, but one was not available. Total shredded sample weight was about 11 lb (5,000 g) with an average bulk density of 89.8 lb/ft³ (1.44 g/cm³). The shredded scrap was split using a riffle splitter from which analytical samples were taken as well as samples for detinning. The analysis is listed in table 2.
Sample preparation and cell operation with PCS were as follows: Material for testing was riffle-split into samples between 150 and 200 g. Before the scrap was detinned, it was screened to 100 pet plus 20 mesh to prevent the fines from falling through the wire mesh basket. The fines (<1 wt pct of the sample) were discarded. Stripping time (equation 1) was based on the Sn content of the post-screened weight, assuming an average of 0.30 wt pct Sn (Ccs) and a current density of 60 A/ft². The final Sn content of the detinned scrap varied between 0.035 and 0.009 wt pct with an average of 0.014 for 32 tests. The treated scrap’s Sn content did not increase greatly as the tests proceeded, showing no inefficiency with time.
Impurity buildup from detinning PCS was also studied during these tests. The concentrations of Cu, Fe, and Pb were determined in the electrolyte and in the Sn deposit. Lead was the highest Sn deposit impurity, present up to 0.15 wt pct. This value remained relatively constant, while the lead in the electrolyte increased linearly up to 0.223 g/L during the 32 detinning test runs. The same trend was observed with Fe. The Fe contamination of the Sn deposit never exceeded 0.05 wt pct, while the Fe concentration in the electrolyte increased linearly up to 1.62 g/L during the 32 runs. Copper was detected in very small quantities (0.0007 g/L) in the electrolyte. The small level present (<0.2 wt pct) in the Sn deposit would not significantly affect the value of the Sn product.
The cell operated on a problem-free basis with PCS for 32 consecutive tests, approximately 5 h of operation. During this time, cathode current efficiency remained at approximately 95 pct. Gassing was minimized, and all Sn deposits were rated good to fair on the basis of appearance. A good deposit is smooth and contains no spongy or powdery areas. The Sn electrolyte concentration remained at 28±2 g/L throughout the tests, indicating Sn was not being stripped from the electrolyte.
Detinning Municipal Solid Waste Magnetics
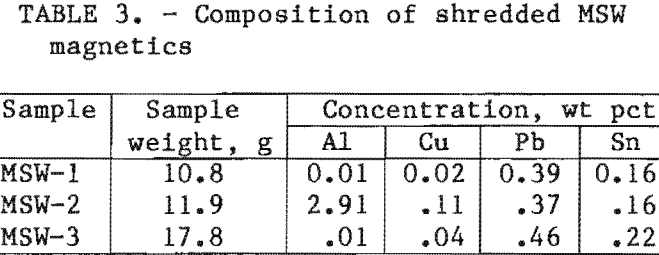
About 100 lb (45.5 kg) of MSW magnetic scrap was obtained from the Resource Recovery Unit of the Ontario Ministry of the Environment in Toronto, Canada. The scrap had already been shredded, magnetically separated, and air-classified. It was reshredded in the same manner as PCS, then again subjected to magnetic separation and air classification to remove Al and organic wastes entrapped in the original sample. Riffle splitting was then done to obtain samples for analysis. Their composition is listed in table 3. The Al and Cu content varied greatly from sample to sample. The variations in Pb and Sn content were small, considering the heterogeneous nature of the material (fig. 3). The average bulk density for this material was 101 lb/ft³ (1.62 g/cm³).

The scrap was blended, coned, and quartered. Then, to have representative sampling, riffle-split portions were taken for the individual tests.
MSW magnetics sample size and preparation, cell operation (current density 60 A/ft²), and starting electrolyte composition were identical to that used when detinning PCS. The initial average Sn content (Ccs) equation 1 was 0.18 wt pct.
The testing of MSW magnetics was divided into four parts. In the first experiments, the shredded MSW magnetics were given no further treatment other than reshredding, magnetic separation, and air classification. In the second, they were chemically delacquered by soaking in acetone for 4 days followed by lacquer thinner for 2 days, washed with fresh acetone, and then air dried. In the third, the MSW magnetics were roasted for 30 min at 350° C. Finally, the MSW magnetics were flash-roasted; i.e., they were placed cold in a furnace already heated to 400°, 500°, or 600° C for residence times of 2, 5, 10, or 15 min, and then removed and allowed to cool. Treatments, Sn content, and cathode current efficiencies are listed in table 4.
The detinning results on all the different pretreatments of MSW magnetics were good, producing acceptable steel scrap for remelting. During most of these tests, cathode current efficiency was above or close to 90 pct, and Sn deposits were of acceptable quality. The average final Sn content of MSW magnetics that had only been shredded and magnetically separated with no further treatment was 0.017 wt pct. None of the pretreatments appear to significantly affect Sn removal.
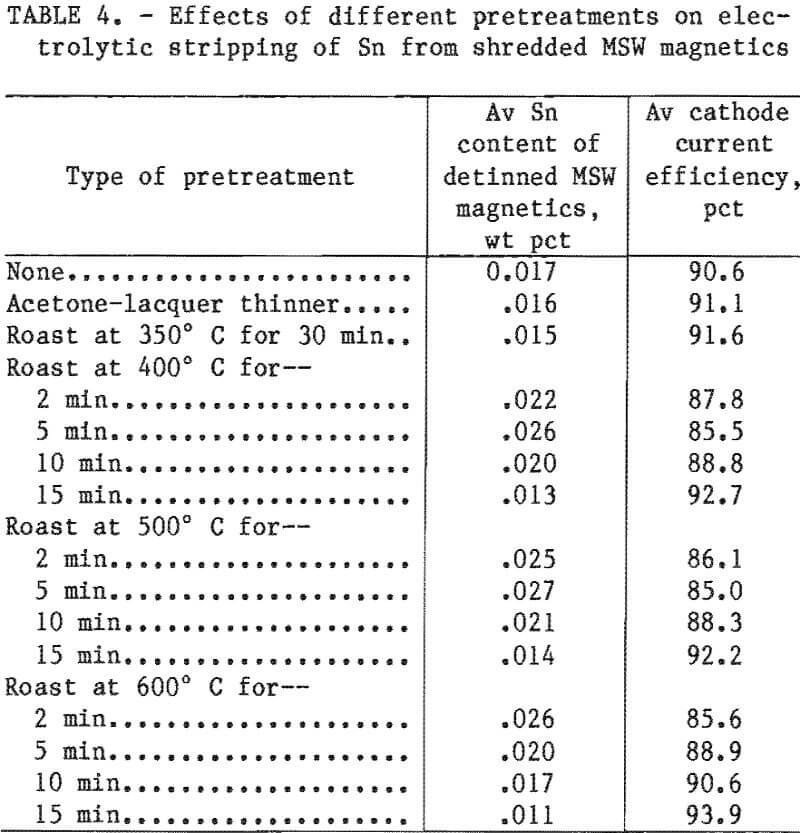
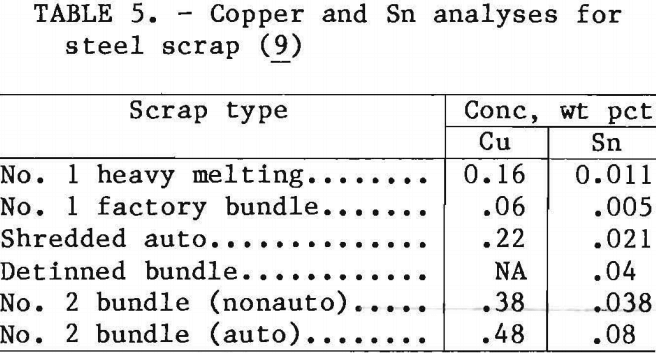
Table 5 lists Cu and Sn values for some of the grades of steel scrap that are currently marketed. The fluoborate detinned MSW appears comparable to detinned bundle or No. 2 bundle scrap. A representative Pb analysis for shredded auto scrap is 0.01 wt pct. Detinned MSW would contain larger amounts of Pb.
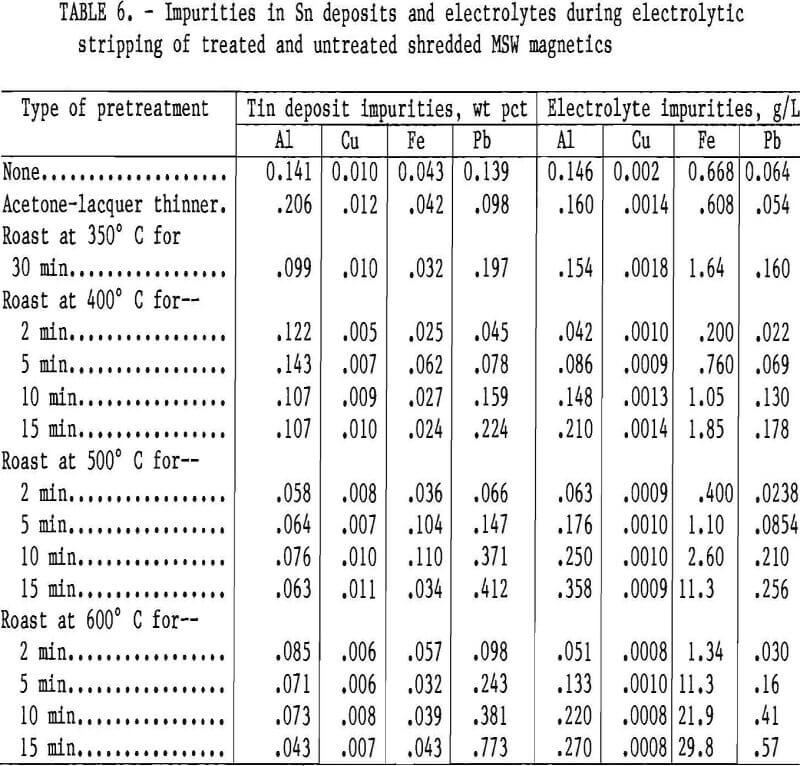
Analytical data for the Sn deposit and electrolyte are listed in table 6. The impurity levels varied depending on the type of pretreatment. All Sn deposits contained less than 1.0 pct total impurities. Aluminum and Pb were the most abundant elements in the Sn deposits but were found in lower concentrations than Fe in the electrolyte. Copper content was small in both the Sn deposits and the electrolyte, but the deposits’ Cu content was significant considering the amount of Cu in the electrolyte.
Pretreatment of the MSW with acetonelacquer thinner before detinning did not significantly affect either the Sn deposit or the electrolyte. Roasting did not appear to significantly improve deposit quality, and many of the roasts were deleterious. The Fe content in the electrolyte increased when roasted scrap is used, probably owing to the formation of rust (iron oxide). Also, Pb concentration increased in both the electrolyte and the Sn deposit when roasted scrap was detinned.
The metal produced met the ASTM specification for Grade E Sn (99 pct Sn). This material is used in cast bronzes, bearings, and Pb-base alloys. Its Al content makes it unacceptable for use in solder and tinning.
Summary
Samples of PCS and MSW magnetics were detinned using an electrolytic process. The electrolyte composition chosen for the research was 60 to 65 g/L HBF4, 25 to 30 g/L Sn, 6 g/L gelatin, and 1 g/L beta naphthol. At a current density of 60 A/ft², the cathode current efficiency was usually above 90 pct. Aluminum, Cu, Fe, and Pb impurities had little or no effect on cathode current efficiency, but significant amounts of Cu and Pb codeposited with the Sn. The detinned scrap appeared comparable to several commercial grades of steel scrap. The Sn recovered met the ASTM specification for Grade E Sn. Additional pretreatment to remove lacquer coating did not improve Sn quality or detinned product quality.
