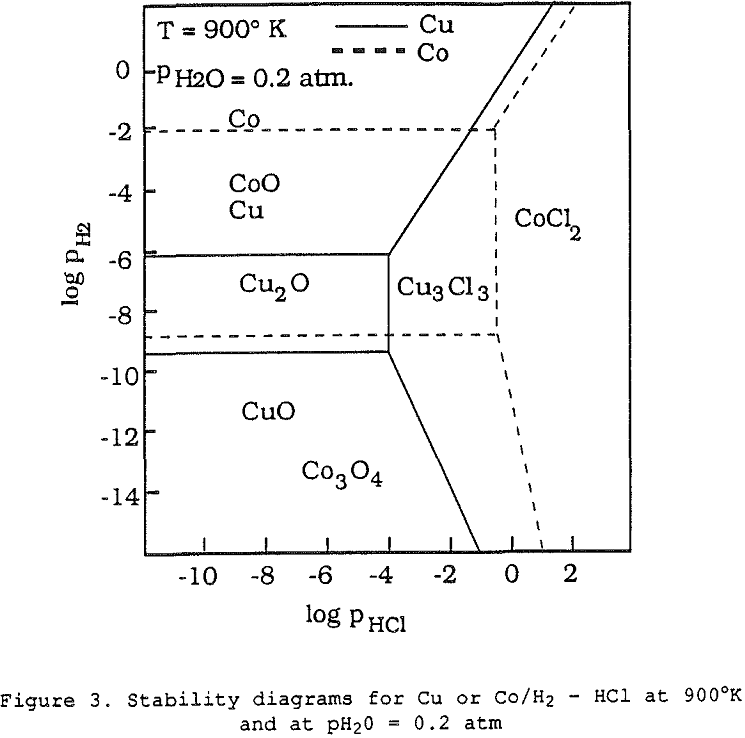The segregation process has been known for many years and applied industrially for the treatment of various refractory copper ores. The principles of the process are briefly reviewed; the process was tested in the laboratory on two copper-cobalt oxide ores from Gecamines, Shaba Province, Zaire. Various parameters of the process, such as segregation temperature, dosage of reducing and chloridizing agents and flotation conditions were investigated.
Segregation Roasting – Thermodynamics and Kinetics of the Process
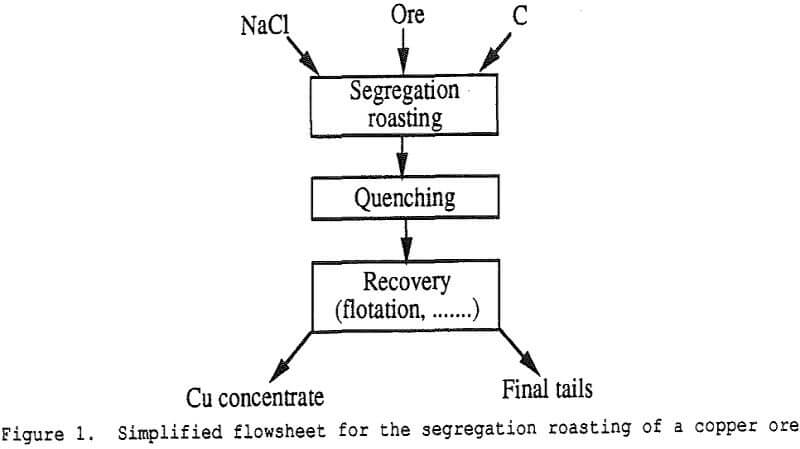
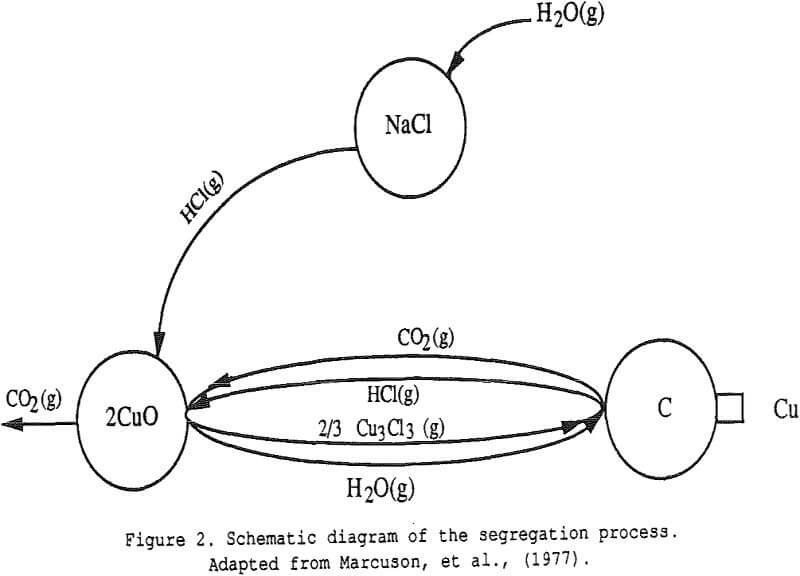
When a copper ore is heated at the proper temperature with appropriate amounts of sodium chloride and carbon, it is observed that the copper is first transformed into volatile chlorides which are then reduced to copper metal onto the carbon particles – hence the name segregation process.
Step 1 : Generation of Chloridizing Gases
Step 2 : Formation of Metal Chlorides
Step 3 : Volatilization of the Metal Chloride
Step 4 . Reduction of the Metal Chloride on the Carbon Particle
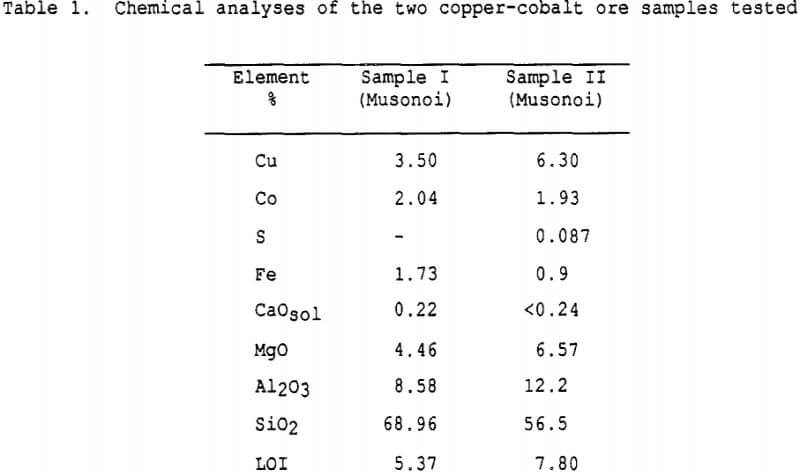
Two samples of copper-cobalt oxide ore from a Gecamines operating mine (Musonoi pit) were studied during the investigation.
Both ore samples contained copper mostly as malachite (Cu(OH)2 · CuCO3) and cobalt as heterogenite (CoO·OH). The gangue was mostly constituted by quartz, with clays, chlorites and limonite.
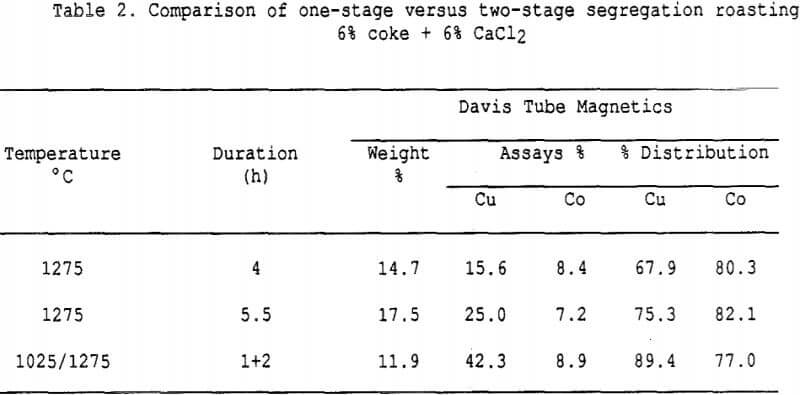
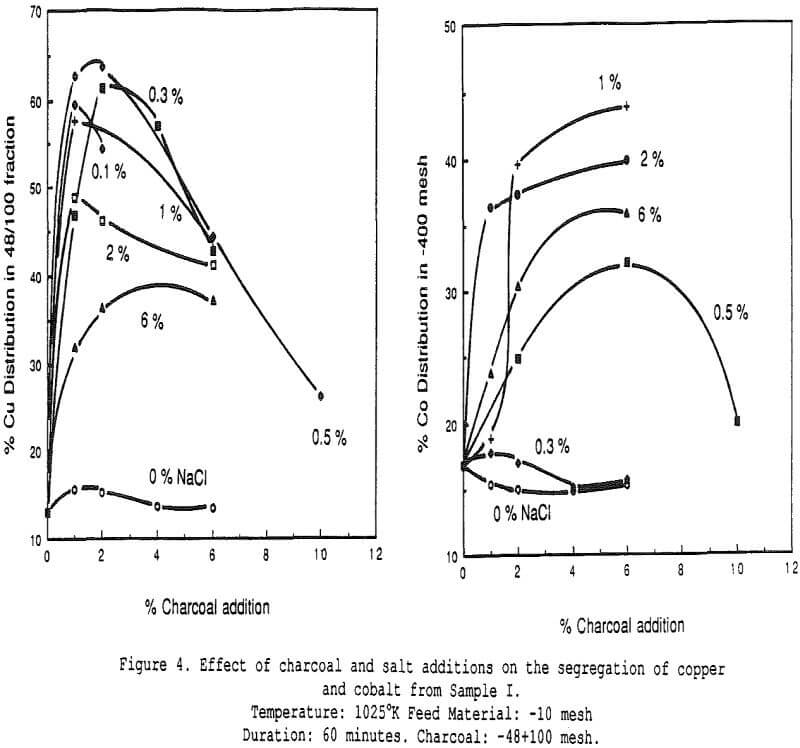
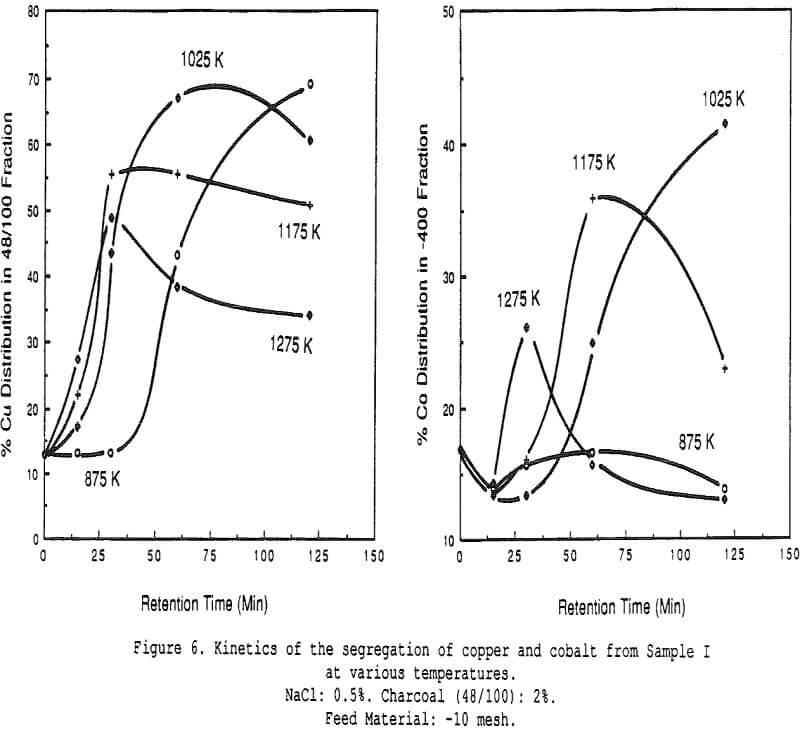
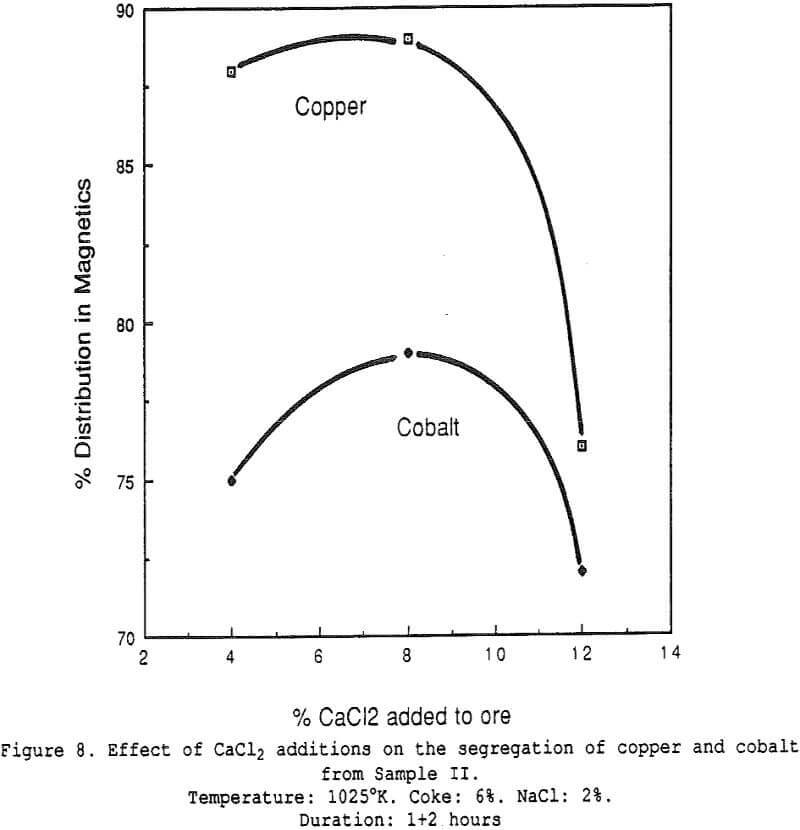
Effect of Charcoal Addition – Some general segregation roasting results obtained on sample I.
The amount of copper recovered with the charcoal increases significantly with small additions of salt. All curves show the presence of an optimum charcoal dosage, which indicates that a compromise must be sought between segregating conditions and in-situ reducing conditions (when too much charcoal is added).
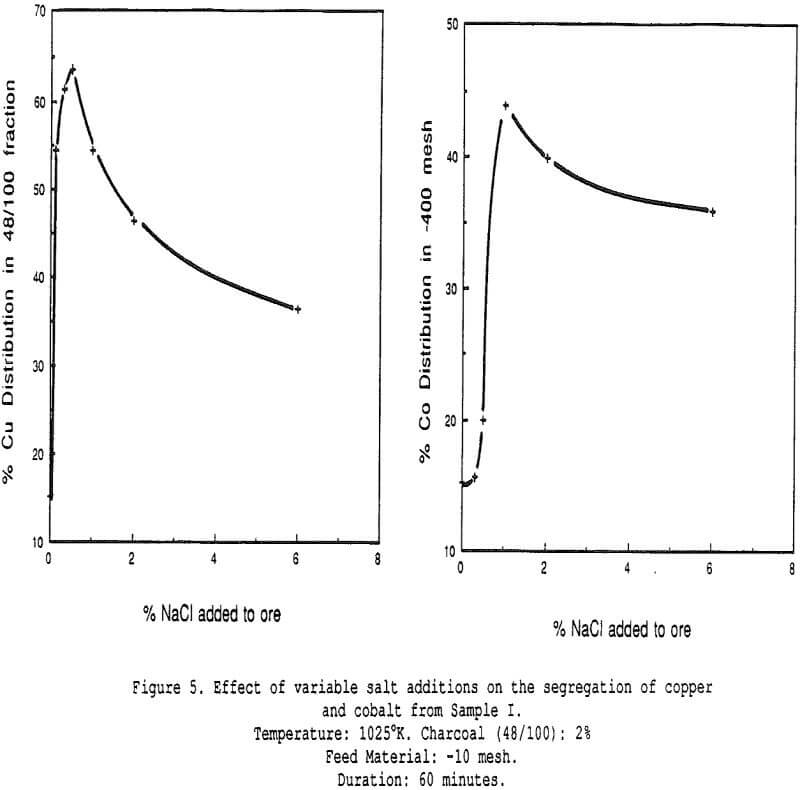
Effect of Salt Additions – The effect of varying the amount of salt added for the same ore sample. The presence of an optimum is clearly visible, both for the segregation of copper onto the charcoal and the migration of cobalt towards the fine fraction.
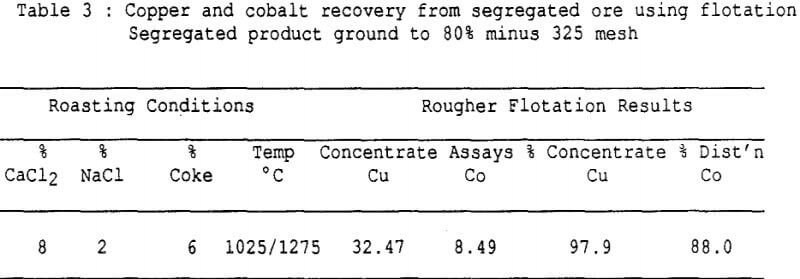
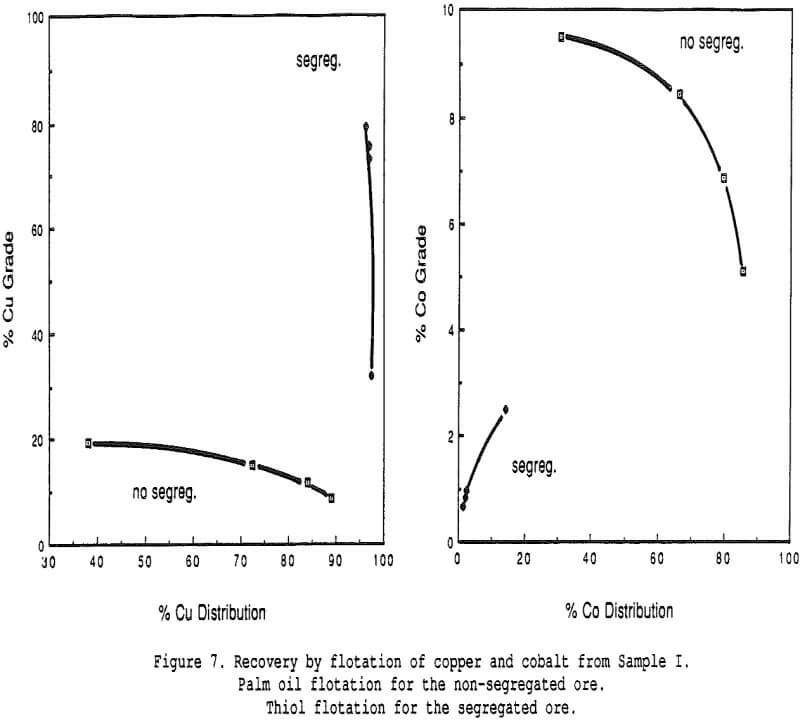
Recovery of Copper and Cobalt – An attempt was made to recover the copper and the cobalt from a segregated ore using a standard flotation method with a thiol collector. Grade-recovery curves are illustrated and compared with grade-recovery curves produced by a typical fatty acid float (palm oil process) on the same ore before segregation. The recovery of copper proceeded extremely well (more than 95% recovered in a 79% Cu concentrate) but the cobalt was completely left behind.