Table of Contents
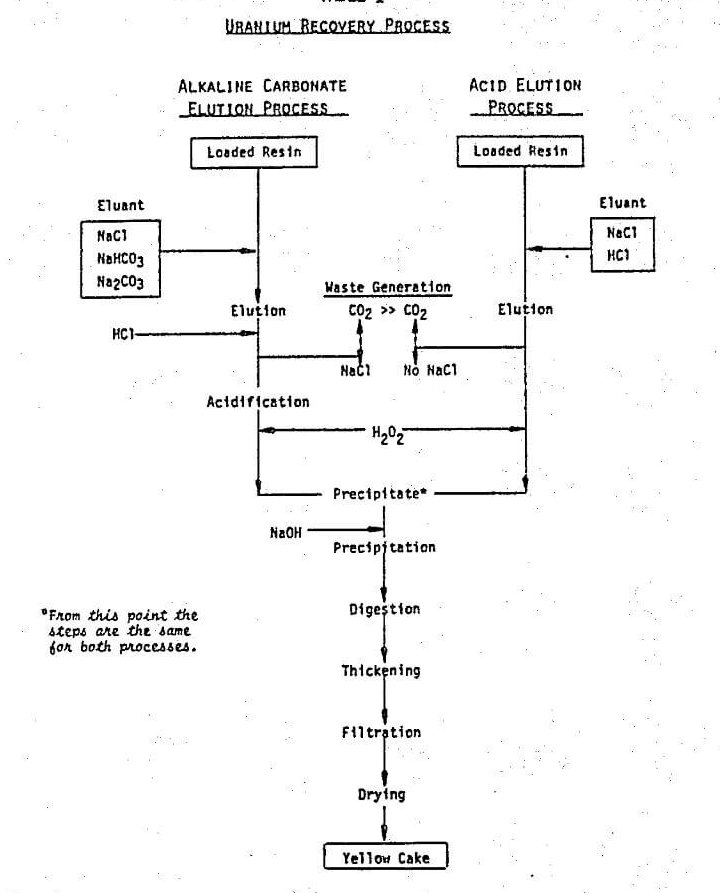
An effective technique has been developed to elute ion exchange resins loaded in the carbonate leaching process for uranium recovery. The procedure involved elution with about 0.5 to 2 bed volume of 36.5 g/l. HCl, followed with 5 to 10 bed volume of acidic solution containing 3.65 g/l. HCl and 58.5 g/l. NaCl. This procedure has been patented.
Laboratory experiments have been conducted to show that the technique is better than the conventional elution procedure using basic solution. The advantages of the procedure are:
- Sharper elution, leading to eluate of higher uranium concentration
- More complete elution, leading to increased resin loading capacity and decreased loading leakage
- Reduced acid and eliminated carbonate consumption
- Reduced waste for disposal.
Besides the extensive laboratory tests, the technique has been successfully demonstrated in the field. It is compatible with the plant designed for conventional alkaline elution and lends itself to commercialization.
The objective of this study is to develop a process which is more effective than the current procedure and also compatible with a conventional plant. Specifically, the objectives of the process are:
- Reduction of acid consumption to save chemical costs and to minimize the bleed stream and waste disposal problems. Waste disposal could be a crucial problem in many parts of the world.
- Elimination of carbonates to decrease chemical costs and simplify the process.
- Maintain ion-exchange resin performance at its fresh state to give high loading capacities and diffusion rates, and low uranium leakage.
- Minimise elution tailing.
Chemistry of acid elution
It is generally believed that “In alkaline circuits the uranium is adsorbed as the uranyl tricarbonate anion, and the use of an acidified eluant will result in the evolution of carbon dioxide, possibly disrupting column operation or causing rupture of the resin beads”. Consequently, the conventional approach to elution of uranium in the carbonate leaching process is to use sodium chloride with addition of sodium carbonate and sodium bicarbonate to prevent hydrolysis and remove the last 2 or 3 percent of the uranium contained in the resin. However, in order to fulfill the above objectives, a different approach is needed. Despite the warning in Reference, direct elution using dilute acid solution 0.1 N HCl (Table 1) in lieu of NaCl/NaHCO3/Na2CO3 was attempted.
The overall reaction involved in the acid elution is:
R4UO2(CO3)3+6H+ → R4 +4 + UO2+² +3CO2↑+3H2O…………………………….(1)
where R is the anionic resin.
The uranyl tricarbonate complex is exchanged from the resin and decomposed irreversibly to generate CO2 and uranyl cation. The uranyl cation is no longer exchangeable with anionic resin, rendering the elution effective. In other words, the elution is effected by decomposing the uranyl tricarbonate complex with acid to form uranyl cations that prevent back exchange and minimize the tailing. This is contrary, in principle, to the carbonate elution process which depends entirely on the mass action law:
R4UO2(CO3) 3+4A- ↔ R4A4+ UO2(CO3)3 -4…………………………………………………….(2)
where A- are anions in the solution, such as Cl-, HCO3- and CO3-.
In the acid elution process, NaCl in the eluant shifts the equilibrium shown in eq. 3 to the right to improve the elution operation. The eluted uranyl carbonate complex is finally decomposed irreversibly into CO2 and uranyl cation (eq. 4).
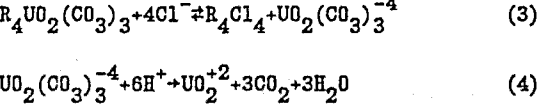
Minimization of waste disposal
When the eluant contains a substantial amount Cl-, the ion exchange between Cl- and UO2(CO3)3 -4 complex take place (eq. 3). Thus, the decomposition of UO2(CO3)3 -4 and CO3= occurs in the liquid phase rather than in the resin pores, so that the rapid generation of CO2 will not lead to resin breakage. It is desirable to maintain the level of Cl- as high as possible to improve elution (eq. 3) and to minimize the size of the bleed stream. Since the generation of Cl- per cycle is fixed, the higher the concentration of NaCl in the system, the smaller the bleed stream it requires to control the ion concentrations below the desired level. The concentration of NaCl permissible will be dictated by both physical considerations (e.g., too high a density to make upflow elution difficult) and chemical considerations (e.g., formation of UO2Cl4- which is difficult to elute and could possibly contaminate the yellow cake).
Minimization of acid consumption
A further advantage of direct acid elution is a reduction in total acid consumption. The conventional carbonate process requires an acidification step after elution, in which the uranyl tricarbonate complex is decomposed prior to yellow cake precipitation. While the acid consumption for this decomposition is essentially the same as that in acid elution, extra acid is consumed in decomposing the carbonate and bicarbonate used in the elution step. The acidification step in the carbonate elution process is, of course, eliminated when the acid process is used.
Improving performance of ion exchange resin
The calcite deposit on the resin is decomposed by the acid according to eq. 5:
CaCO3 + 2H+ → Ca ++ + CO2↑ + H2O……………………………………………..(5)
Since the resin is cleaned and regenerated each cycle, this regeneration is less harmful (shock) to the resin, and the resin is kept in a fresh state for continuously optimal performance.