Table of Contents
The chemicals involved in the work of this paper utilize a combination of mechanisms: charge effect neutralization and polymer bridging. There are a number of trends in the results that support this dual mechanism. The slow settling rate and insensitivity of changes in coagulant concentration are the typical effects of compounds used for charge neutralization. A positive influence from increasing the molecular weight of the coagulant was also observed although the molecular weight influence is not necessarily as important as chemical molecular modification. The settling rate dominance of the flocculant bridging mechanism was also obvious. The positive influence of increasing flocculant molecular weight would also be expected.
The presence of a strong bridging mechanism reduces the level of coagulant required and, to some extent, reduces the differences between types of coagulant chemicals. In the coal suspensions of this study, initiation of the solids concentration process by a small amount of prefloc treatment (bridging) is more efficient than a pure coagulation approach. Once bridging is established, water quality can be controlled by the concentration of coagulant. This is often the case for relatively dilute slurries but does depend on the nature of the suspended material. Also, the relative sensitivity of coagulant concentration to ash content is expected because of the higher charge effects and surface area associated with ash materials. Although slurries not requiring charge neutralization can be treated with flocculant alone, a combination flocculant/coagulant system provides better plant economics with the slurries of this study than either flocculants of coagulants alone.
Cylinder Settling Tests
Because this type of testing is simple and quick, it is well suited for preliminary screening of a large number of chemical combinations and concentrations. Cylinder tests are also readily adaptable to on-site flocculant evaluation using fresh refuse. The flocculants to be tested are mixed at 0.25% (by weight) and diluted to 0.025% immediately before use. The following evaluation procedure was used.
- Two hundred fifty cc of refuse slurry was poured into each 250 cc test cylinder.
- An incremental amount of 0.025% flocculant test solution was added to the refuse slurry, and the cylinder was inverted four times.
- The coagulant was added; the cylinder was inverted four times and then placed in front of a light source.
- The unhindered settling rate of the solid/ liquid interface is determined first. At the end of three minutes the turbidity of the supernatant liquid and the height of the compacted solids is measured.
Laboratory Cylinder Settling Tests
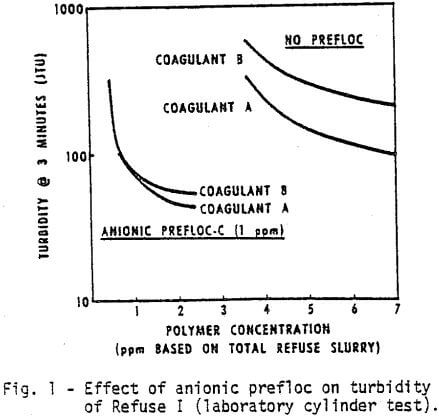
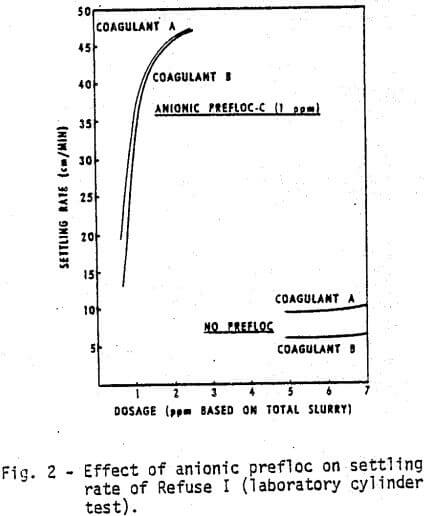
The list of chemicals utilized in this study is given in Table I. The first test was to compare in the laboratory Coagulant A, a new cationic polyelectrolyte with Coagulant B. Figures 1 and 2 demonstrate, respectively, the water clarity and settling rates observed on Refuse I at various coagulant concentrations with and without a flocculant pretreatment (prefloc) of 1 ppm Flocculant C.
A number of conclusions are evident:
- Coagulant A performs consistently better than Coagulant B either with or without prefloc treatment.
- The use of a prefloc treatment with 1 ppm of Flocculant C significantly lowers the dosage required of either coagulant, and also significantly enhances the performance of either coagulant.
- The cost of obtaining the same water clarity with a prefloc treatment is less than half the cost of a straight coagulant system.
- The use of Flocculant C alone, as inferred by the anionic prefloc data at very low concentrations of coagulant, will not provide satisfactory clarity with this refuse.
- The anionic polymer dampens the differences due to coagulant type.
On-Site Cylinder Settling Tests
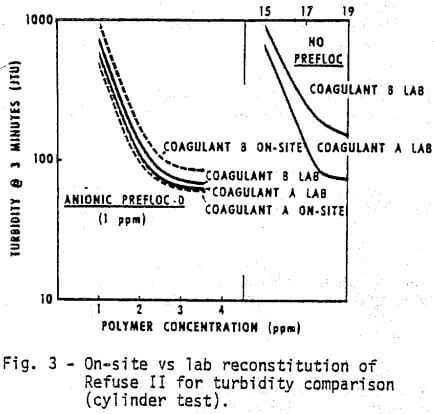
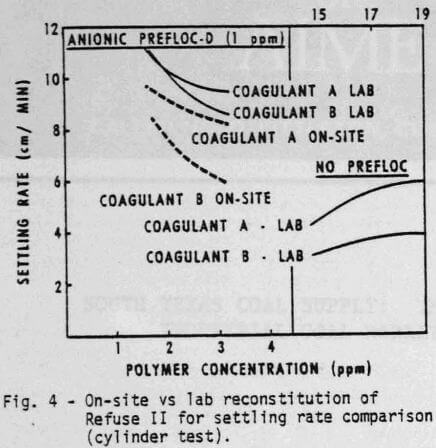
Changes in refuse will produce substantial changes in the response to the chemical system. Figures 3 and 4 demonstrate, respectively, the water clarity and settling rates observed on a different feed, Refuse II. Two or three times as much coagulant is required with Refuse II (Figure 3) to produce the same clarity obtained with Refuse I (Figure I). The composition and amount of ash in the feed to the thickener as well as the type of water are obvious reasons for variations. The refuse used in all the laboratory tests was reconstituted at 5% dry solids. The amount of solids going to the thickener for the on-site comparisons (Figures 3 & 4) was variable but did approximate 5%. The ash content of Refuse I (Figure 1 & 2) was 13%, while the ash content of Refuse II (Figures 3 & 4) was 75% to 80%.
A number of conclusions are evident:
- Coagulant A again performs consistently better than Coagulant B either with or without the prefloc treatment.
- Without a prefloc treatment, the concentration requirement of either coagulant alone is considerably higher with Refuse II than Refuse I indicating the influence of higher ash content.
- Again, the presence of a prefloc treatment (Flocculant D) coupled with a coagulant significantly reduces the quantity and cost of either coagulant system alone.
- The relative ranking of Coagulant A and B obtained from cylinder settling tests in the lab is the same as that obtained with on-site cylinder tests.
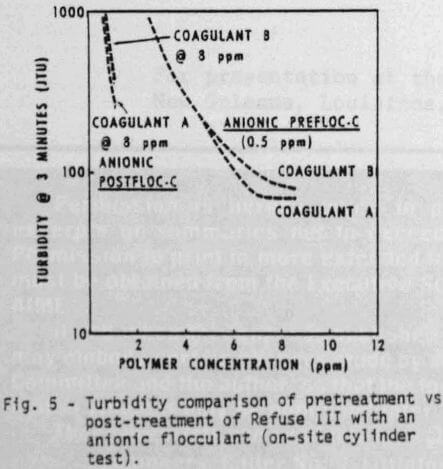
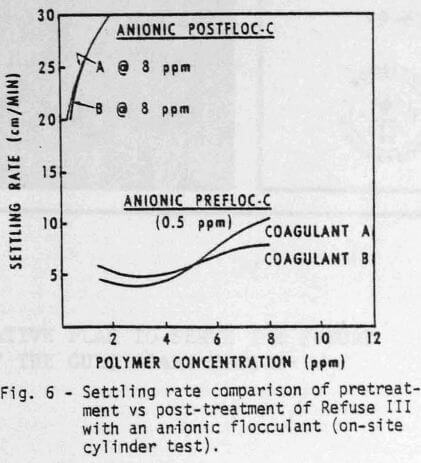
Cylinder settling tests can also be used to determine the best polymer addition sequence. Although the effect of addition sequence may not be apparent in all cases, it is very important with Refuse III (Figures 5 & 6). For example, at equal dosage of the coagulants (8 ppm), satisfactory clarity (Figure 5) and adequate settling rate (Figure 6) can be obtained by using ½ ppm anionic polymer as a pretreatment. Even higher dosages of anionic flocculant as a post-treatment would not provide satisfactory water quality.
 |
 |
 |
 |
 |
 |
 |
 |

