Table of Contents
Recent studies suggest that toxic and highly persistent poly-and perfluorinatedalkyl substances (PFAS) are much more prevalent in tissue and soil than in water. The increasing length of perfluoroalkyl chain in PFAS is correlated strongly to lower solubility/higher adsorption behaviour of a particular PFAS molecule in the environment. This poses a significant challenge to developing analytical methods, especially for the extraction of PFAS from solid matrices. The adsorption and mobility of PFAS (perfluoroalkyl chain length C6-C14) through soil were investigated by rinsing a soil column with 60 ml spiked rainwater at pH 4, pH 10 and pH 5.3. PFAS which adsorbed onto the soil column were extracted using a conventional vortex/sonication method. Aqueous eluate and extracts were analyzed using LC-MS/MS and quantified using an internal standard method. PFAS with chain length C6-C9 migrated completely or partially through the column and were effectively extracted from soil with 100% recovery. However, long-chain PFAS (C12-C14) did not appear to migrate through the column and had less than 50% recovery from the soil. The same extracted soil was then subjected to high-pressure accelerated solvent extraction (ASE) which yielded 100% recovery for long-chain PFAS. By extension, the application of ASE for the extraction of PFAS from mussel tissue and cooking oil was also investigated. Oil samples were spiked at 10 ng/g and tissue samples spiked at 25 ng/g. These samples were incubated and then subject to ASE. Recoveries of PFAS from these matrices were acceptable for all analytes and were highest for C11-C14. Blanks contained no significant amounts of PFAS. The use of ASE for extraction of longer chain perfluoroalkyl acids (C16, C18), sulfonates and sulfonamides from solid matrices was also investigated. The results of this study will inform our understanding of how to evaluate the transport of PFAS through soil and the assessment of PFAS bioaccumulation in tissue and food.
Abstract
Recent studies suggest that toxic and highly persistent poly-and perfluorinatedalkyl substances (PFAS) are much more prevalent in tissue and soil than in water. The increasing length of perfluoroalkyl chain in PFAS is correlated strongly to lower solubility/higher adsorption behaviour of a particular PFAS molecule in the environment. This poses a significant challenge to developing analytical methods, especially for the extraction of PFAS from solid matrices. The adsorption and mobility of PFAS (perfluoroalkyl chain length C6-C14) through soil were investigated by rinsing a soil column with 60 mL spiked rainwater at pH 4, pH 10 and pH 5.3. PFAS which adsorbed onto the soil column were extracted using a conventional vortex/sonication method. Aqueous eluate and extracts were analyzed using LC-MS/MS and quantified using an internal standard method. PFAS with chain length C6-C9 migrated completely or partially through the column and were effectively extracted from soil with 100% recovery. However, long-chain PFAS (C12-C14) did not appear to migrate through the column and had less than 50% recovery from the soil. The same extracted soil was then subjected to high-pressure accelerated solvent extraction (ASE) which yielded 100% recovery for long-chain PFAS. By extension, the application of ASE for the extraction of PFAS from mussel tissue and cooking oil was also investigated. Oil samples were spiked at 10 ng/g and tissue samples spiked at 25 ng/g. These samples were incubated and then subject to ASE. Recoveries of PFAS from these matrices were acceptable for all analytes and were highest for C11-C14. Blanks contained no significant amounts of PFAS. The use of ASE for extraction of longer chain perfluoroalkyl acids (C16, C18), sulfonates and sulfonamides from solid matrices was also investigated. The results of this study will inform our understanding of how to evaluate the transport of PFAS through soil and the assessment of PFAS bioaccumulation in tissue and food.
Spiking
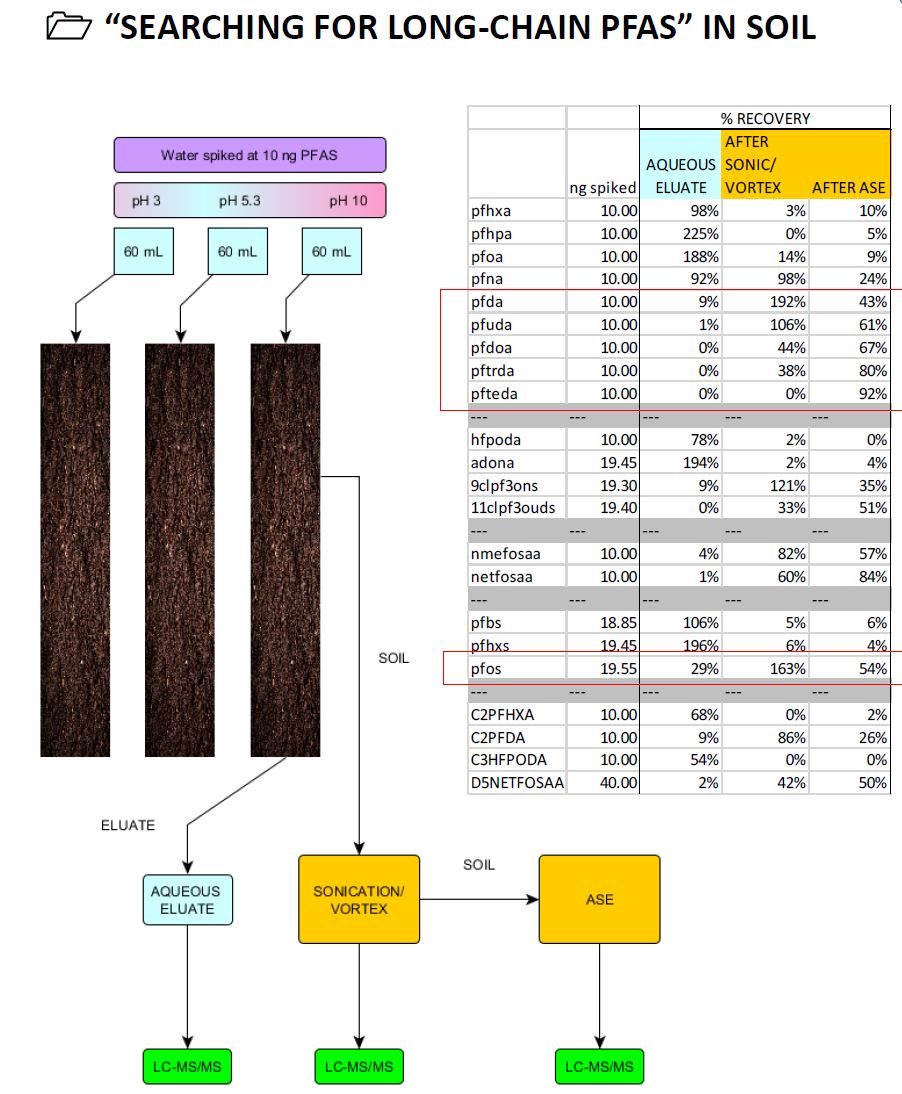
- SOIL
10 g soil was washed through with 60 mL water at pH 4, pH 5.3 and pH 10, spiked at 10 ng PFAS. 4 spikes and 4 blanks were processed. - MUSSEL TISSUE
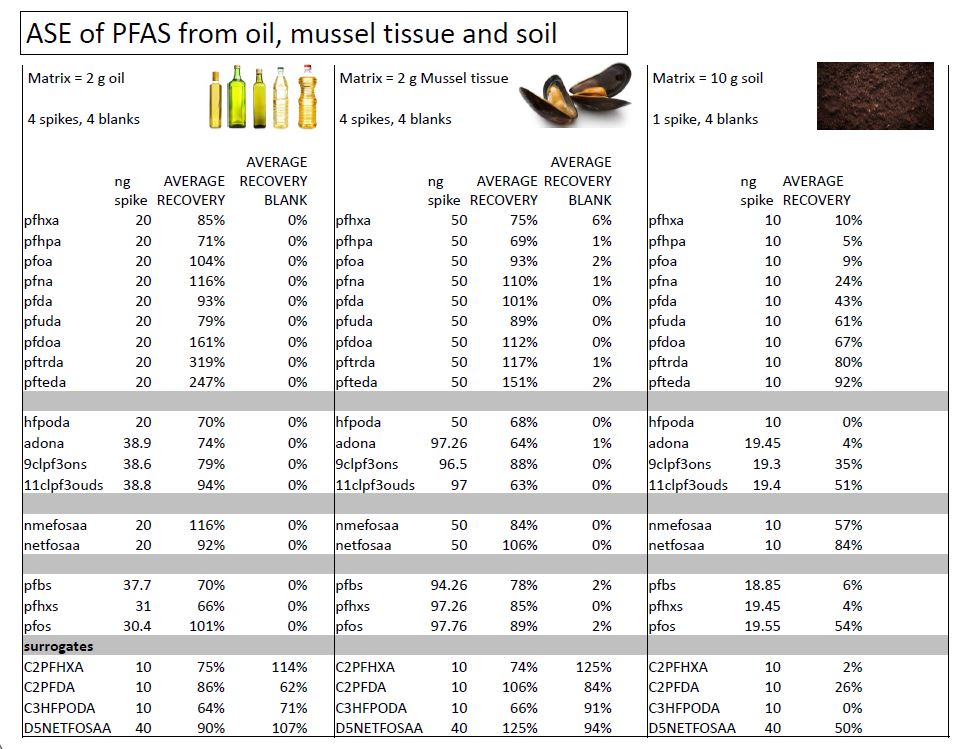
2 g mussel tissue was spiked with 50 ng PFAS. 4 spikes and 4 blanks were processed and analyzed. - COOKING OIL
2 g canola oil was spiked with 20 ng PFAS. 4 spikes and 4 blanks were processed and analyzed.
Extraction
- SONICATION/VORTEX: Solid sample is mixed with 1:1 methanol:water, sonicated and vortexed for 5 minutes.
- ACCELERATED SOLVENT EXTRACTION Solid sample is mixed with methanol and subject to methanol extraction under high pressure and temperature.
Results:
- pH has no significant effect on the adsorption of PFAS to soil under the conditions investigated.
- C6 to C8 PFAS migrated completely or partially out of the soil column and were present in the aqueous eluate
- C10 to C14 PFAS did not appear to migrate through the soil under the conditions investigated and were not present in the aqueous eluate.
- C11 to C14 PFAS were extracted in under 50% recovery from the soil using a conventional sonication/vortex method
- C11 to C14 PFAS were extracted fully from soil using accelerated solvent extraction (ASE)
- All PFAS tested, including C10-C14 PFAS, were also extracted in high yield from tissue and cooking oil using ASE.
Conclusion:
Accelerated solvent extraction (ASE) is an effective technique for extracting a variety of PFAS, most notably long-chain PFAS > C11, from several solid matrices. The use of ASE for extraction of longer chain perfluoroalkyl acids (C16, C18), sulfonates and sulfonamides from solid matrices was also investigated and continued the trend of high extraction of long-chain PFAS using ASE. The results of this study will inform our understanding of how to evaluate transport studies of PFAS through soil and the assessment of PFAS bioaccumulation in tissue and food.
https://www.pacificrimlabs.com/
About the author:
“Matthew MacLennan is Senior Scientist and Director of LC/MS Method Development at Pacific Rim Laboratories in Surrey, British Columbia, Canada. He has obtained ISO 17025 accreditation for PFAS testing in water and soil according to EPA 537.1 and ASTM methods. Matthew has 5 years experience analyzing complex contaminant mixtures such as naphthenic acids and oil sands process-affected water using capillary electrophoresis, TOFMS and Orbitrap MS, working on conjunction with Environment Canada. Matthew obtained his PhD in Analytical Chemistry from the University of British Columbia in Vancouver, BC, Canada.“
