The following minerals could be plainly seen in the ore: magnetite, pyrites of iron, pyrites of copper, quartz, and garnet. Neither galena nor zinc-blende were visible, but the ore contained a trace of lead, and in some samples of ore from the Muertos mine, 1.19 per cent, of zinc was found. Antimony and arsenic were not present. Native gold could scarcely be seen in specimens of the ore, and it only appeared—mainly in extremely fine particles—after pulverizing and washing. Magnetite was in every instance the most prominent mineral, its quantity varying between 43 and 67 per cent. Determinations of copper and sulphur showed that from 3.5 to 7 per cent, pyrites of copper, and from 3 to 22 per cent, pyrites of iron were present.
The gold-value of the ore varied between 0.3 oz. and 0.8 oz. per ton. I do not speak of specimens, but of average samples of large quantities of ore put through the battery. In ore that had been crushed through a No. 40 screen, the gold appeared, in part, as free gold, the rest being thoroughly mixed with the magnetite and pyrites, both minerals containing about the same percentage of gold. If this free gold was brought in contact with pure quicksilver it did not amalgamate readily, even if the quicksilver was charged with sodium amalgam.
All the gold-ores contained some silver, partly alloyed with the native gold, partly mineralized. But the silver-value was so low that it had to be entirely neglected in the metallurgical treatment.
That the gold-ores of Las Minas, on account of their pyritic character, were not fit for raw amalgamation, could be seen a priori, and the practical tests made at the mill had fully established this fact. Such ores can only be prepared for subsequent beneficiation by a complete roasting. Hence I directed my first efforts to roast the ore dead. By that I do not mean only a desulphurization of the pyrites, and a decomposition of the sulphates formed in roasting, but also a higher oxidation of the magnetite to ferric oxide.
Experiments in Roasting:
The ore was crushed wet by stamps (this being the only means of pulverizing at my disposal) with No. 40 screen on the mortar, and allowed to settle in a tank. In the sluice-boxes, between the battery and the settling-tank, the coarser particles of the native gold were caught. This arrangement was made in order to avoid errors of sampling, produced by coarse gold, in subsequent metallurgical experiments.
The roasting was done in a small reverberatory furnace. A charge of 500 pounds was all the furnace could conveniently take, but generally not more than 200 pounds were roasted, this quantity being more than sufficient for my metallurgical experiments.
Charge No. 1.—San Anselmo ore, with 64 per cent, magnetite. The charge contained, after 4½ hours’ roasting, 44.8 per cent, magnetite. The solution, from a sample leached with water, gave no copper-reaction, the copper sulphate being already decomposed. Some aluminium sulphate was found. After 14½ hours the magnetite was only reduced to 42.3 per cent. After 16½ hours one-half of the ore was discharged. It still contained 39.6 per cent, magnetite.
Charge No. 2.—To the ore remaining in the furnace, 2½ per cent, of salt was added, and the charge stirred for 1 hour. It was now drawn into a pile and left in the furnace, firing being discontinued. After 4 hours the ore contained 20.7 per cent, magnetite, and after 8 hours 19.2 per cent. The ore was discharged after 22 hours. The magnetite had increased to 24.5 per cent. A sample, tested for copper, showed that in one ton of ore 0.44 pound of copper was present as cupric chloride, and 1.32 pounds as cuprous chloride.
Charge No. 3.—Muertos ore, with 47 per cent, magnetite. The charge contained, after 5 hours’ roasting, 35.7 per cent, magnetite, and considerable copper sulphate. After 7 hours 30.5 per cent, magnetite was found, and the copper sulphate had been entirely decomposed. After 17 hours one-half of the ore was discharged. It now contained 20.0 per cent, magnetite, no copper sulphate, but some aluminium sulphate.
Charge No. 4.—To the ore left in the furnace, 3½ per cent, salt was added, and treated like Charge No. 2, with this difference, that the furnace was kept hot by continued firing. After 3 hours the ore contained 6.2 per cent, magnetite, and much copper chloride. After 5 hours the magnetite had increased to 10.7 per cent., remaining at the same figure after 8 hours. The fire in the furnace was now stopped, and the ore discharged after 15 hours. It contained 11.8 per cent, magnetite. The cupric chloride had entirely disappeared, and only 0.6 pound of copper was present as cuprous chloride in one ton of ore.
Charge No. 5.—San Anselmo ore, with 65.7 per cent, magnetite. Mixed at once with 5 per cent, salt.
After 4 hours’ roasting, a sample contained 1.6 per cent, magnetite, and considerable cuprous chloride. After 7 hours the magnetite had increased to 2.6 per cent. Firing was now stopped, the charge drawn into a pile, and left for 13 hours in the furnace. After discharging, the magnetite had increased to 5.6 per cent. Very little cuprous chloride was present, hardly sufficient for determination. A soluble aluminium salt was still left in the ore.
Charge No. 6.—San Anselmo ore, with 67.2 per cent, magnetite. Mixed at once with 5 per cent. salt.
After 1½ hours the magnetite had decreased to 18.6 per cent.; after 4½ hours, to 1.4 per cent. After 5½ hours the ore was discharged. It now contained 2.5 per cent, magnetite. In one ton of ore there were present 0.2 pound of copper as cupric chloride, and 0.6 pound of copper as cuprous chloride.
Summary of Results:
These experiments present many interesting features. They show:
First.—That a mere oxidizing-roasting leaves a large percentage of the magnetite intact, and that a continuation of the process is of very little benefit after the pyrites have been roasted dead. Some aluminium sulphate, resulting from decomposition of the garnet, remains undecomposed, a salt that would not interfere with any treatment to which the ore may be subjected.
Second.—A direct chloridizing-roasting removes the magnetite almost completely and very rapidily.
Third.—If the chloridizing-roasting is continued beyond a certain time, magnetite is re-formed, cuprous chloride becoming oxidized by withdrawing oxygen from ferric oxide. This is very evident in Charge No. 5.
Loss of Gold in Chloridizing-Roasting:
It remains, however, to record the most remarkable results of these experiments. In determining the gold value of the ore, before and after chloridizing-roasting, I found an enormous loss of this metal by volatilization. These results were so entirely unexpected that I first concluded an error had been made in the assays. Such was not the case, and in repeating the experiments, by roasting in a muffle, the facts were established beyond any doubt. In the latter case well-mixed samples were carefully assayed, and then one assay-ton mixed with three per cent, salt, roasted for one hour, and assayed after roasting.
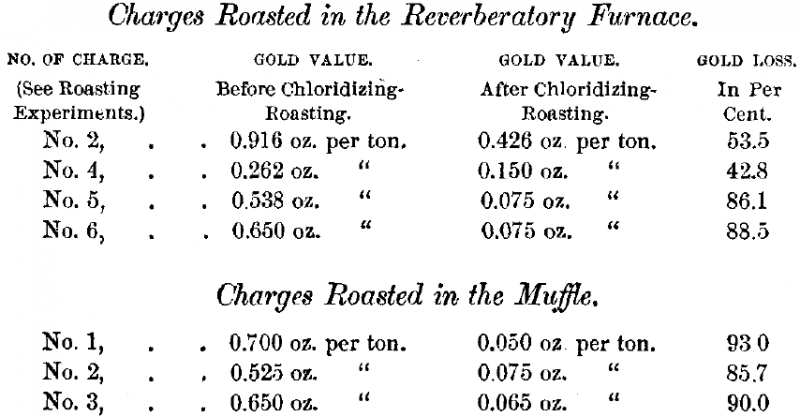
There is no doubt that the volatilization of the gold takes place with that of the copper chlorides. The loss increased with the quantity of these chlorides formed and then volatilized.
This is shown by the much smaller loss in the reverberatory furnace charges, No. 2 and No. 4, in which the salt was added after a complete oxidizing-roasting, thereby reducing the formation of the copper chlorides to a minimum. Temperature and time of roasting must also influence the result.
That copper chloride is by no means an essential element in producing this loss is shown in the following experiments, made by Mr. C. Butters, with a gold-ore entirely free from copper. The gangue of the ore was a very hard whitish quartz, intimately mixed with about 7 per cent, calcite. It contained 5.55 oz. silver, and 0.65 oz. gold per ton. In ordinary specimens neither silver minerals nor gold were visible to the naked eye. After concentrating a quantity of the ore, pulverized so as to pass a No. 40 wire-screen, and examining the concentrates under a powerful microscope, a dark steel- gray silver mineral was revealed, covered with minute crystals of gold. In several samples examined, all the gold appeared to be attached to the silver mineral, and none was seen by itself.
Besides, there was some pyrites of iron. In subjecting the ore to an oxidizing-roasting it assumed a pinkish color, and lost about 3 per cent, in weight, corresponding with the amount of carbonic acid driven out from the calcite. A loss in silver from 2 to 9 per cent, took place, but none in gold.
Very different were the results in chloridizing-roasting. These experiments were also conducted in the muffle, at a cherry-red heat. In roasting several samples for 1 hour with 5 per cent, salt, from 70 to 80 per cent, of the silver, and from 68 to 85 per cent, of the gold were lost. The chloridized ore assumed a dead-white color. After leaching it with water, the solution gave only a faint chlorine-reaction, the salt having been completely volatilized, and with it the larger portion of the precious metals. Roasting with 10 per cent, salt did not increase the loss in silver and gold.
The ores in both experiments were of an uncommon and peculiar character and contained the gold in exceedingly fine particles.
While I am not prepared to say, upon experimental proof, in what form the gold is volatilized in chloridizing-roasting, I would suggest that it escapes as a double salt. If this is the case, the loss will depend—other circumstances being the same—upon the volatility of the chloride with which the gold chloride is combined. Now, the chlorides of copper are much more easily volatilized than sodium chloride, and the loss should be greatest in case copper is present in the ore, as is actually shown by my experiments.
Plattner speaks of the loss of gold in oxidizing-roasting, but entirely neglects chloridizing-roasting. G. Kuestel records a loss of 8 percent, of gold in roasting telluret-ores with salt, and states that by increasing the temperature and time of roasting, the loss in gold may be 20 per cent, and more. Mr. R. P. Rothwell has drawn my attention to C. H. Aaron’s book on leaching gold- and silver-ores. Aaron suffered heavy losses in the chloridizing-roasting of gold- bearing sulphurets, and in making tests in a muffle, lost more than 50 per cent, of the gold. Both Kuestel and Aaron neglect to give details of their experiments. In Australia, the chloridizing- roasting of gold-ores was abandoned on account of heavy losses.
I have repeatedly expressed the opinion that the volatilization of metals in roasting is principally a function of time. My experiments in the chloridizing-roasting of silver-ores have established this fact in the case of silver. Why should it not hold good with regard to gold? The advantages of instantaneous roasting, as done in the Stetefeldt furnace, are as yet very little understood and appreciated by metallurgists. It is erroneously assumed that this system, for which the Germans have coined the word Staubstromrostung, is only of very limited application. Leaving all economical and mechanical questions out of consideration, the system of instantaneous roasting has three great advantages, namely:
1st. The loss of precious metals is reduced to a minimum.
2nd. The roasted ore is in the most favorable condition for subsequent treatment.
3rd. The fine ore-particles accumulating in the dust-chambers are thoroughly roasted.
The last-named point, being one of the essential features of the Stetefeldt furnace, is too well known to require any discussion. To ascertain the loss of precious metals by experiments on a large scale in a direct way, namely, by weighing and sampling the ingoing raw ore and the outgoing roasted ore, is attended with so much inconvenience and expense, that I have not found parties in charge of any mill employing Stetefeldt furnaces willing to undertake it. Hence I had to get at the question by an indirect method. If a sulphuret ore is subjected to an oxidizing-roasting, the outgoing elements are generally of greater weight than the ingoing ones, and the result is a loss in weight. The same is the case in chloridizing-roasting, provided we compare the weight of the raw ore, without salt, with the weight of the roasted ore after leaching out the soluble salts. Under the circumstances it follows that the assay-value of the roasted ore must be greater than that of the raw ore, in proportion to the loss in weight the latter has suffered, provided no silver has been volatilized. If, however, the roasted ore should fail to come up to the required value, no other inference can be drawn but this: that silver has been lost in roasting.
In 1880, I carried out at the Ontario mill, Utah, under circumstances that are not likely to occur again, a series of comparative experiments in the Stetefeldt and Howell furnaces. In one of these experiments the ore treated by both furnaces was exactly the same. It came from the same stamp-battery, and, after being elevated to a hopper, was distributed from the spout of a conveyer-trough, one- half going to the Stetefeldt and the other half to the Howell furnace. The latter had been provided with a system of dust-chambers as complete and well-constructed as that attached to the Stetefeldt furnace. The roasted ore was discharged into cars and carefully sampled. At the end of the experiment, the dust-chambers were cleaned out. The average result, after a run of six days, was as follows:
Value of raw ore, ……………………………………………………………………………..106.0 oz. silver per ton.
Value of roasted ore, after leaching with water:
From Stetefeldt furnace, …………………………………………………………………..112.5 oz. silver per ton.
From Howell furnace,……………………………………………………………………….98.1 oz. silver per ton.
Difference: 14.4 oz. silver per ton, or 13.5 per cent, of the silver in the ore. This difference represents the loss of silver by volatilization in the Howell furnace above that in the Stetefeldt furnace. That the latter must be very small is evident from the much higher value of the roasted ore compared with the raw ore. The increase in value corresponded very nearly with the loss in weight, as ascertained by muffle-tests, which the raw ore suffered in roasting. In these experiments, from 25 to 28 tons of ore were roasted per day, and divided between the two furnaces, running them only at half capacity. In regard to the Howell furnace, this had the effect of keeping the ore much longer in the cylinder than if the latter had received its full charge of 25 tons. Subsequently the Howell furnace was kept running for 10 days at full capacity, receiving the ore from 20 stamps, and the Stetefeldt furnace was supplied from another battery of 20 stamps. The results from the Howell furnace were as follows:
Value of raw ore,……………………………………. 120.6 oz. silver per ton.
Value of roasted ore,………………………………..115.5 oz. silver per ton.
Difference,……………………………………………..5.1 oz. silver per ton.
or the roasted ore was 4.2 per cent, lower in value than the raw ore. The results from a 40 days’ run of the Stetefeldt furnace were as follows:
Value of raw ore,……………………………………. 109.8 oz. silver per ton.
Value of roasted ore,………………………………..115.8 oz. silver per ton.
Difference,……………………………………………..6.0 oz. silver per ton.
or the roasted ore was 5.4 per cent, higher in value than the raw ore. This makes a difference of 9.6 per cent, between the two furnaces, and represents the loss of silver in the Howell furnace above that taking place in the Stetefeldt furnace. In comparing the losses of silver resulting in the two experiments, we have again a confirmation of my theory of the influence of time. When the Howell furnace was running at half its capacity, and the ore remained longer in the cylinder, the loss in silver was 3.9 per cent, greater than in the second experiment, when the furnace was run at full capacity. The character of the Ontario ore is such that these losses are by no means astonishing. It contains nearly all its silver as a fahl-ore, rich in antimony and arsenic, and carries a large percentage of zinc- blende. In conducting the process in the Howell furnace, it was necessary to roast at a slightly higher temperature than in the Stetefeldt furnace. With a low fire, the ore had a tendency to run forward in the cylinder, and get discharged while still partly raw. The average value of the tailings resulting from the amalgamation of the roasted ore was so nearly uniform that any difference was within the limit of errors in sampling. This shows that the roasting was equally well done in every instance.
At the Lexington Mill, Butte, Montana, a silver-ore that contains a considerable amount of gold is subjected to a chloridizing-roasting in the Stetefeldt furnace. The ore is composed of silver-bearing pyrites of iron, pyrites of copper, zinc-blende, galena, and some native silver, with a quartz gangue, sometimes replaced by silicate of manganese. Antimonial and arsenical silver-minerals are absent. Native gold is not visible. From the character of this ore, we can safely infer that it will lose less silver in roasting than Ontario ore, and that in a Stetefeldt furnace this loss must be exceedingly slight. Now if, in the roasted ore, the proportion of silver and gold remains the same as in the raw ore, it follows that also the loss in gold must be very small. I give below such statistics as are in my possession, but will take up this subject again when I can supply more facts.
The average of about 600 samples, taken at the Lexington mill, from October, 1883, to August, 1884, and compiled from the monthly averages of the assayer’s report is as follows:
Raw ore,……………………………………………………………………………………..45.9 oz. silver; 0.77 oz. gold per ton.
Roasted ore, after leaching out soluble salts,……………………………………. 50.0 oz. silver; 0.84 oz. gold per ton.
Increase in value,………………………………………………………………………….4.1 oz. silver; 0.07 oz. gold per ton.
Increase in value,………………………………………………………………………..8.8 per cent, silver; 9.1 per cent. gold.
From this it would appear that the loss of gold in chloridizing- roasting in the Stetefeldt furnace is somewhat less than that of silver. The difference, however, in the increase of value between silver and gold is only 0.3 per cent., and within the limits of errors in assaying.
Finally, I wish to say a few words about the advantage of instantaneous roasting in relation to a peculiar condition of the roasted product. The most striking facts I have already presented in my paper on Russell’s Process. I refer to paragraph 19, which contains the experiments in lixiviating ores subjected to an oxidizing-roasting. If ores were roasted in the muffle, the amount of silver that could be extracted by hyposulphite solutions decreased rapidly with an increase of time in roasting. If, however, the ore had been roasted in a Stetefeldt furnace, as much as 85.7 per cent, of the silver could be extracted by Russell’s process. It is not to be assumed that different combinations of silver are formed by the instantaneous process of roasting, but that the silver compounds formed are more easily soluble on account of their short exposure to heat. We know, for instance, that peroxide of iron becomes quite insoluble in dilute acids after being exposed to a high temperature for a long time. The same is the case, but less pronounced, with other oxides. Instances of this kind might be multiplied.
Experiments in Amalgamation:
Taking into consideration the low grade of the Las Minas gold- ores, the high price of salt and chemicals in Mexico, and the local resources and facilities of the country generally, I became convinced that extraction of the gold by amalgamation, after previous oxidizing- roasting, would be the only economical process. With this object in view, the following experiments were undertaken. In experimenting with such gold-ores there were only two ways possible: either to work very large quantities, or to make tests on such a small scale that the results possessed analytical exactness. The latter course was the only one that could be pursued. These experiments being somewhat novel and surprising in their results, it will be necessary to enter into details. The apparatus used consisted of one iron mortar, five inches in diameter, with a rather flat bottom (F. M.); one iron mortar, five inches in diameter, with a concave bottom (C. M.); and one wedgewood mortar, with concave bottom (W. M.). In grinding the charges, the iron mortar with flat bottom proved to give superior results as compared with the iron mortar with concave bottom—its grinding effect was the best. The iron mortars were used to imitate the work of an iron pan; the wedgewood mortar that of an arrastra. The charges consisted of five assay-tons of roasted ore (1 A. T.=29.166 gm.). This was ground or mixed wdth water and quicksilver, the latter being added either immediately or after the ore had been treated with other reagents (10 gm. quicksilver to a charge of 5 A. T. of ore).
The charges were amalgamated either at ordinary temperature or with heating. The latter method had no beneficial effect.
After the amalgamation had been completed, the charge was thinned with water, and gently stirred, until the quicksilver was united. For separating the tailings from the quicksilver, an enameled iron pan (8 inches diameter on the top, 6 ¼ inches diameter at the bottom, 1½ inch height) was used. With a little skill and practice, with the aid of potassium cyanide, if necessary, the quicksilver was easily gathered in one mass, and transferred to a beaker-glass. It was now dissolved in nitric acid. Here the following phenomena were observed : In case only a trace of copper, and very little silver had been amalgamated, the gold remained at the end of the operation as a spongy globule that could be easily transferred to a drying-cup. The gold so obtained was, however, not perfectly pure. It had to be packed into a piece of lead-foil,inquartated with silver, cupelled, and the alloy redissolved in the customary way. If considerable copper had been taken up in amalgamation, the gold appeared as an exceedingly fine powder—after solution of the quicksilver. It became necessary to filter the solution, collect the gold on the filter, incinerate the latter, and cupel the ashes as above. The presence of a large amount of silver produced a similar but less pronounced effect, and also in this case filtering was necessary. It will be seen that these tests must give a positive and very accurate result. That in every instance the gold-value of the charges was ascertained in the most careful manner, and all reagents tested for their purity, it is hardly necessary to mention. In all, fifty-one charges were amalgamated, and those giving the most interesting results are recorded below:
No. 1.—Roasted San Anselmo ore, from charge No. 1 of “roasting experiments.” It contained 39.6 per cent, magnetite. No copper sulphate present. The charge had been roasted for 16½ hours. Time of amalgamation 3 hours, with quicksilver added at once. Time of settling ½ hour. (Time of settling the same in all subsequent experiments.)
Value of ore, 0.916 oz. gold per ton.
Extracted, 0.195 oz. gold per ton, or 21.2 per cent.
No. 2.—San Anselmo ore, from charge No. 5, roasted chloridizing. It contained 5.6 per cent, magnetite, and a very slight amount of cuprous chloride. Quicksilver added at once. Time of amalgamation, 2½ hours.
Value of ore, 0.075 oz. gold per ton.
Extracted, 0.060 oz. gold per ton, or 80.0 per cent.
No. 3.—San Anselmo ore, from charge No. 1, sifted through a No. 80 screen. It contained 26.6 per cent, magnetite. No copper sulphate. Grinding, 1 hour; then quicksilver added. Time of amalgamation, 2½ hours.
Value of ore, 0.450 oz. gold per ton.
Extracted, 0.125 oz. gold per ton, or 27.7 per cent.
No. 4.—The same ore as in No. 3.
Grinding, 1 hour; then 1.5 gm. mercuric chloride added, and a few drops hydrochloric acid (Designolle process), and worked for ¾ of an hour; then quicksilver added, and amalgamated ¾ of an hour longer.
Value of ore, 0.450 oz. gold per ton.
Extracted, 0.345 oz. gold per ton, or 76.6 per cent.
In repeating these charges (Nos. 3 and 4), but without previous grinding, adding quicksilver in one case, and mercuric chloride in the other at once, and amalgamating for 2½ hours, ordinary amalgamation extracted 31.1 per cent., and the Designolle process 82.2 per cent, of the gold.
Further experiments with the Designolle process, in which the mercuric chloride was reduced to 0.5 gm. per charge of 5 A. T., gave less favorable results, extracting only from 51 to 56 per cent, of the gold.
All the above charges were worked in iron mortars.
No. 5.—Blanket-washings,from San Anselmo ore,roasted oxidizing for 4 hours, contained 19.5 per cent, magnetite. Copper sulphate completely decomposed. (These blanket-washings were obtained in crushing 70 tons of San Anselmo ore for the purpose of sampling it.)
In order to imitate the working of a pan by first grinding without quicksilver, and then amalgamating with raised muller, the ore was ground in the iron mortars for 1½ hours; then quicksilver added, and for 1 hour amalgamated by stirring with wooden pestles.
Value of ore, 1.48 oz. gold per ton.
Extracted in F. M., 0.82 per ton, or 55.4 per cent.
Extracted in C. M., 0.75 per ton, or 50.6 per cent.
In the following charges the same ore was ground for ¾ of an hour, and then quicksilver added and amalgamated with iron pestles, under grinding, for 1½ hours, with these results:
Extracted in F. M., 1.40 oz. gold per ton, or 94.6 per cent.
Extracted in C. M., 1.09 oz. gold per ton, or 73.3 per cent.
Finally, the quicksilver was added at once, and the charges amalgamated for 2½ hours with grinding.
Extracted in F. M., 1.42 gold per ton, or 95.9 per cent.
Extracted in C. M., 1.23 oz. in gold per ton, or 83.1 per cent.
No. 6.—San Anselmo ore, roasted oxidizing for 4 hours. The raw ore contained 67 per cent, magnetite, and the roasted ore 31 per cent. Immediately after discharging no copper sulphate was present, but after cooling it appeared to some extent, undoubtedly in consequence of the presence of aluminium sulphate.
Quicksilver being added to the charge, the amalgamation was continued for 2½ hours.
Value of ore, 0.800 oz. gold per ton.
Extracted in F. M., 0.355 oz. gold, or 44.3 per cent.
Extracted in C. M., 0.340 oz. gold, or 42.5 per cent.
No. 7.—The same ore as in No. 6 was treated in this experiment. But, in place of quicksilver, pure silver amalgam was added (a piece the size of a pea) and the charge ground for 2 hours. Then quicksilver was added, and amalgamated ½ of an hour longer, to collect the amalgam, before settling was commenced.
Value of ore, 0.800 oz. gold per ton.
Extracted in F. M., 0.620 oz. gold, or 77.5 per cent.
Extracted in C. M., 0.565 oz. gold, or 70.6 per cent.
The silver amalgam in the last experiment was moist. In using silver amalgam that was much drier, but by no means hard, the following results were obtained :
Extracted in F. M., 0.676 oz. gold per ton, or 84.0 per cent.
Extracted in C. M., 0.660 oz. gold per ton, or 82.5 per cent.
No. 8.—Muertos ore, roasted oxidizing. Contained 43 per cent, magnetite before, and 19.5 per cent, after roasting. This ore was discharged, by mistake, too soon. A considerable part of copper sulphate had remained undecomposed. Two charges were amalgamated, one in the iron mortar with flat bottom, and the other in the wedgewood mortar, both with silver amalgam. Time of amalgamation, 2½ hours; then quicksilver added and amalgamated ½ of an hour longer.
Value of ore, 0.370 oz. gold per ton.
Extracted in F. M., 0.120 oz. gold, or 31.6 per cent.
Extracted in W. M., 0.316 oz. gold, or 83.1 per cent.
The amalgam from the iron mortar was very base, that from the wedgewood mortar very fine.
No. 9.—Muertos ore, roasted oxidizing. Contained 28 per cent, magnetite after roasting. A well-roasted charge, showing not a trace of copper sulphate after discharging. After cooling, a slight quantity of copper sulphate was, however, re-formed.
The amalgamation was carried out with silver amalgam as in previous experiments, but the amalgam squeezed very dry.
Value of ore, 0.340 oz. gold per ton.
(a) Extracted in F. M., 0.296 oz. gold per ton, or 87.0 per cent.
Extracted in C. M., 0.286 oz. gold per ton, or 84.1 per cent.
(b) Extracted in F. M., 0.270 oz. gold per ton, or 79.4 per cent.
Extracted in W. M., 0.332 oz. gold per ton, or 97.6 per cent.
(c)Extracted in F. M., 0.292 oz. gold per ton, or 85.9 per cent.
Extracted in W. M., 0.326 oz. gold per ton, or 95.9 per cent.
If the magnetite was ground sufficiently fine, it did not interfere with good settler work. In all these experiments no flowering of quicksilver took place, in spite of the grinding; it united without difficulty, and a loss was hardly preceptible.
The amalgam from the charges worked in the iron mortars contained some copper, even if the ore was apparently free from copper sulphate, while the amalgam from the wedgewood mortar was always fine, no matter how much copper sulphate had been left undecomposed in the ore.
Summary of Results:
These results are of great interest and importance, and throw considerable light upon gold-amalgamation generally. It has often been found extremely difficult to extract a high percentage of gold from roasted ores by pan-amalgamation, and the theory of “rusty gold” has been extensively circulated in metallurgical literature to explain this fact. It has also been found more difficult to extract the gold from gold-bearing silver-ores by pan-amalgamation, especially if the roasting has been conducted in a reverberatory furnace and not by the instantaneous process. From my experiments it appears that in all these cases the low results are due neither to imperfect roasting nor to the formation of gold combinations which cannot be amalgamated, but to the defects of pan-amalgamation itself. My experiments lead to the following conclusions:
First.—That the ore should be well roasted in as short a time as possible. A complete change of the magnetite to ferric oxide is not necessary, and even a large percentage of this mineral may remain unaltered without producing an injurious effect.
Second.—That copper, if it enters the amalgam, even in small quantities, prevents in a great measure the amalgamation of the gold. Lead will no doubt act still more injuriously. Hence, the amalgamation in an arrastra, where no iron is exposed, will give far better results than if the process is conducted in an iron pan. Considerable copper sulphate may be present in the ore without materially impairing the result in an arrastra.
Third.—The amalgamation of finely divided gold requires friction, and contact alone with quicksilver is not sufficient. But more than that: liquid quicksilver eludes in a great measure the contact with gold, and it is necessary to amalgamate with gold-amalgam (for which silver-amalgam was substituted in my experiments, and should be in starting works on a large scale). This amalgam should not be too soft, so that in the process of grinding liquid quicksilver is not pressed out. I have repeatedly drawn attention to the fact that in amalgamation generally great fineness of the material treated is injurious, and need only to point out the difficulty experienced in working raw slimes, or the fine dust from roasted silver-ores.
Fourth.— Other conditions being equal, the result of amalgamation depends upon the grinding efficiency of the apparatus used. The much better yield in the iron mortar with flat bottom, as compared with the iron mortar with concave bottom, demonstrates this clearly. In grinding effect, the wedgewood mortar was about equal to the iron mortar with concave bottom; but in spite of this, it always yielded higher results than the best grinding iron mortar. Hence the arrastra is, under all circumstances, the best amalgamator. It is hardly necessary to state that the arrastra must be so constructed that iron does not come in contact with the ore.
Fifth.—Experiment No. 5 gives exceptional results. They are, however, explained, if we take into consideration the character of the ore treated. This charge, being a product of concentration, contained most of the gold in comparatively coarse particles, which are readily taken up by quicksilver. In this case, too, grinding was essential, and mere mixing gave low results.
Sixth.—These facts throw some light upon the working of the Designolle process. The small quantity of quicksilver reduced from the mercuric chloride, by contact with iron under friction, produces at once solid gold amalgam, and the latter is really the effective reagent.
The amalgamation of gold-bearing silver-ores which have been subjected to a chloridizing roasting cannot be conducted in an arrastra. In such cases it may be profitable to extract by the arrastra process the gold remaining in the tailings.
That an arrastra, the bottom of which is covered with gold amalgam, is the most effective apparatus for amalgamating gold, has been known to the Mexicans for more than 200 years. I may claim to have rediscovered this fact by scientific research. To my knowledge, no series of experiments has ever been carried out before like that recorded in this paper.
If the Las Minas ores are roasted in a Stetefeldt furnace, and amalgamated in arrastras, there is not the slightest doubt in my mind that from ninety to ninety-five per cent, of the gold can be extracted. Besides, the process is well-suited to the country, and the Mexicans understand its manipulations thoroughly.
Metallurgical Treatment on a Large Scale:
This would comprise the following operations:
First.—Crushing the ore in a rock-breaker.
Second.—Drying the crushed ore.
Third.—Pulverizing by Krom’s improved rolls, and screening through No. 30 wire-cloth.
Fourth.—Roasting in a Stetefeldt furnace.
Fifth.—Amalgamating in arrastras.
Sixth.—Settling in American settlers, such as are used in silver- mills.
Seventh.—Straining the quicksilver, retorting the amalgam, and melting the bullion into bars.
At Las Minas there is ample water-power, and ordinary labor and fuel are very cheap. I have shown, by careful estimates, that under the existing favorable conditions, and with an output of 120 tons per day, the expense of mining and reduction would be as follows:
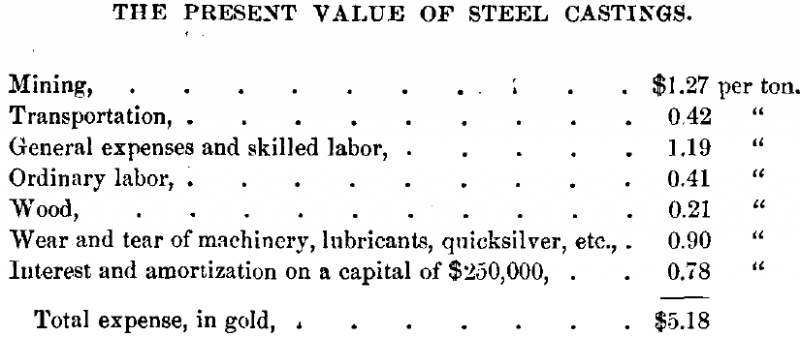
Hence ores of a very low grade can be worked with a profit.
The Amalgamation Of Gold-Ores, And The Loss Of Gold In Chloridizing Roasting, With Especial Reference To Roasting In A Stetefeldt Furnace
By C. A. Stetefeldt, New York City.
