Are you looking for a motor crusher or a crusher motor? This difference is significant. The first is if you want to crush motors, while
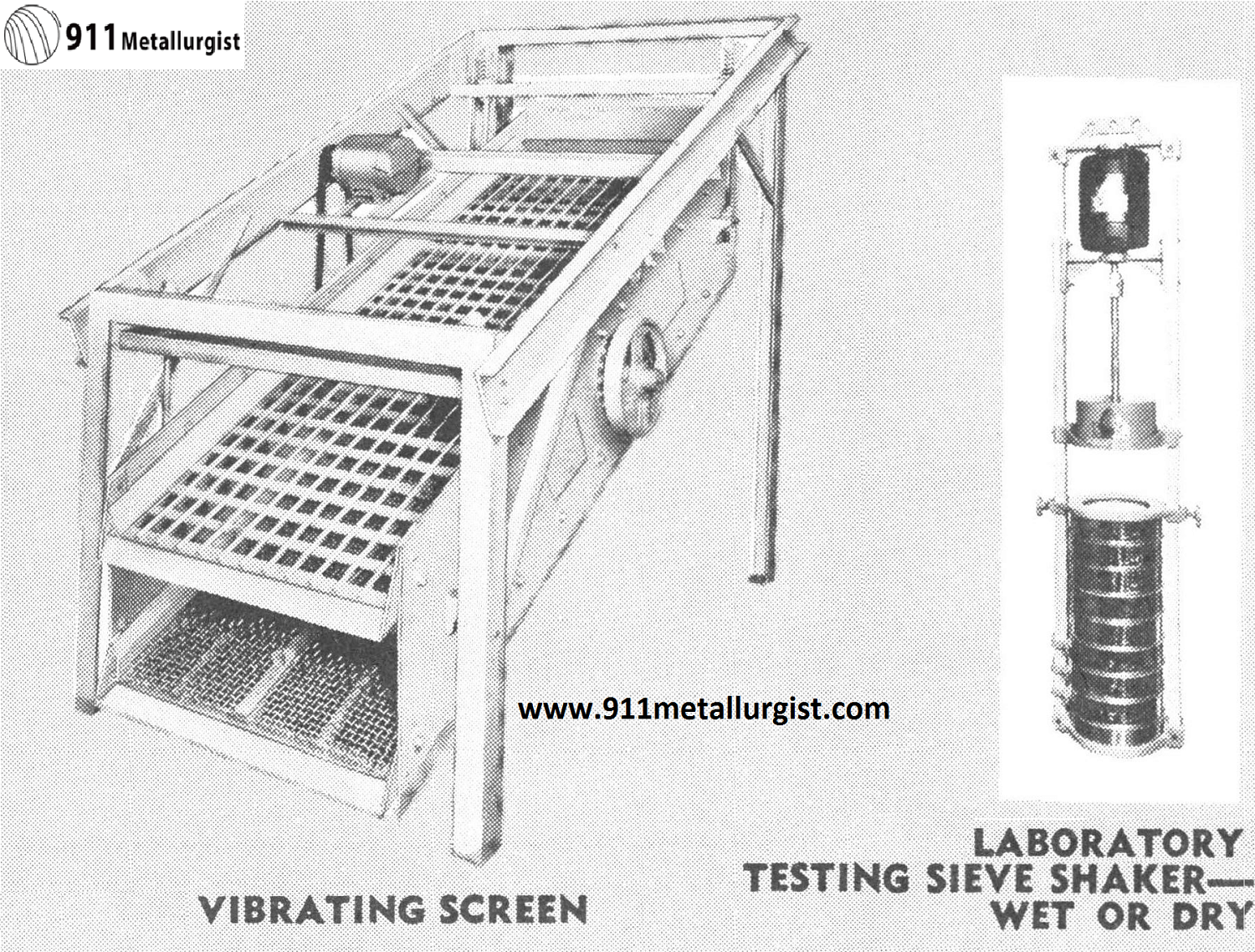
For efficient wet or dry screening—Hi-capacity, 2-bearing design. Flywheel weights counterbalance eccentric shaft giving a “true-circle” motion to screen. Spring suspensions carry the weight. Bearings
How do Seawater Greenhouses Work? A seawater greenhouse is a type of greenhouse technology that utilizes seawater to grow crops in arid or desert regions.
Balancing Resource Extraction and Water Sustainability Lithium, a key component in batteries that power electric vehicles and store renewable energy, has become increasingly vital in
Direct Lithium Extraction (DLE) is an innovative method of extracting lithium from brine or other lithium-rich sources without the need for traditional evaporation ponds. Unlike
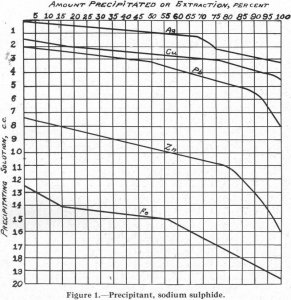
Table of ContentsSolubility of Metal Xanthates in Flotation ReagentsLead, Copper, Zinc, and IronGold and NickelEffect of Surface Oxidation on FloatabilityFlotation Control by Solubility of Metal
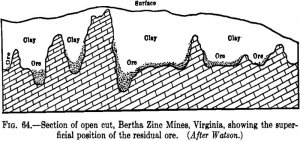
In the examination of an undeveloped prospect a decision must be arrived at from an inspection of the outcrops and the exposures in a few
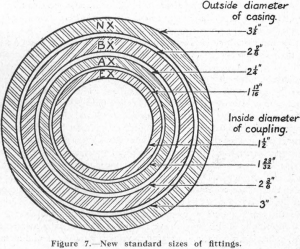
Table of ContentsDiamond DrillsDiamondsUses of the Diamond DrillSupervisionPreparing a Prospect for Diamond DrillingControl of the Location of Drill HolesContracting the DrillingForm of ContractTesting Drill Holes
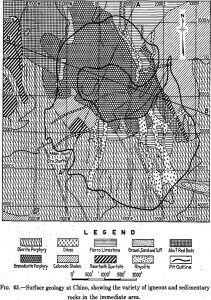
Table of ContentsGenesis of a Typical Porphyry DepositNew CorneliaChuquicamataBraden Frequently the careful engineer puts the word porphyry in quotation marks or precedes it with “so-called,”
Images for illustration purposes only. No rights can be delivered from the illustrations.
911METALLURGY CORP. can not be responsible for errors in typography or photography.
Copyright 2012-2024 911Metallurgist | All Rights Reserved