Minerals have taken their place in the earth’s crust by becoming solid from an originally liquid or, sometimes, gaseous condition, either melted rock matter (magma) or dissolved in water or other solvents. When the change from a liquid to a solid has been slow enough and undisturbed, the minerals have grown as crystals, which are beautiful, smooth forms. Each mineral has its own distinct form; and it is therefore sometimes possible to distinguish minerals one from another by the shape of their crystals, provided the crystals have had a chance to form. That they may grow to perfection, crystals must not be crowded, and they must grow slowly. Where a number of minerals are mixed in the same liquid, each substance goes to its own place, where very small crystals of that particular mineral have started to grow; in this way, there is more or less separation of minerals one from another. Where there has been a very slow growth, very large crystals have been formed, as in the case of the best feldspar deposits, where the separation of quartz from feldspar has been so perfect that great bodies of clean feldspar can be mined.
The various kinds of crystals are distinguished by the angles which their faces make with one another. Though there are thousands of different forms, it has been found that they can all be grouped into six (6) systems. A thorough study of these is too much of a task for a beginner’s course, but the simple introduction that follows will be found useful.
Description of the Crystal Systems
As stated in the last paragraph, the crystal systems are six in number. Any crystal, no matter what its shape, may be assigned to one and only one of these six systems. These systems are differentiated as follows:
Cubic or Regular Crystal Systems
The forms are shaped on the plan of a cube, so that the crystal has the same properties, for example, cleavage, in three directions at right angles to one another.
A crystal may grow faster or slower in one direction than in others. In this way, a cube may become elongated or flattened, so as to appear to belong to the tetragonal or to the orthorhombic System. But its place in the cubic system is shown by the equal brilliancy of its surfaces, equally good cleavage in three directions at right angles to one another, or some other property. Irregular growth may so distort a crystal as to make it look very unlike the perfect form, but the angles between the corresponding surfaces are unchanged (Compare Fig. 6 and Fig. 7).
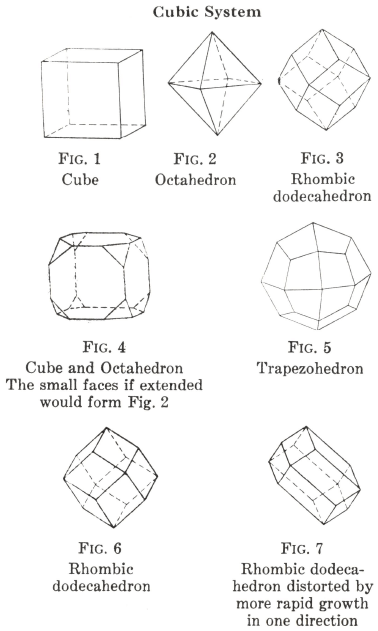
Tetragonal Crystal Systems
Crystals resemble those of the cubic system in being shaped on the square plan; but they have the same properties in only two of the three directions, those for the third being different.
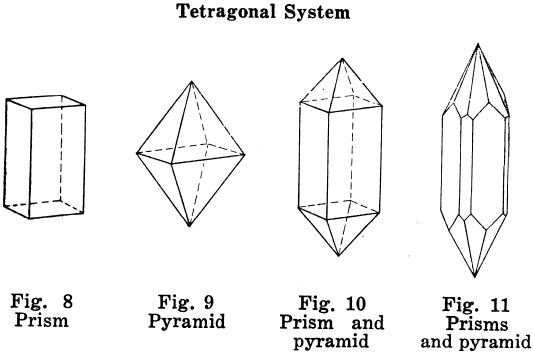
Hexagonal Crystal Systems
Crystals in which angles of 60° and 120° are characteristic; example, the six-side prism.
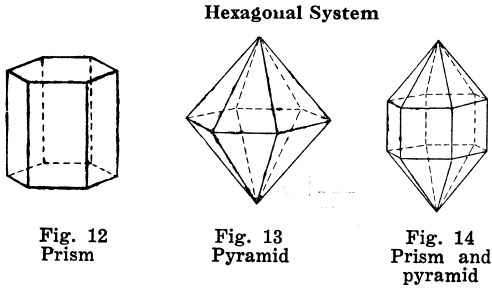
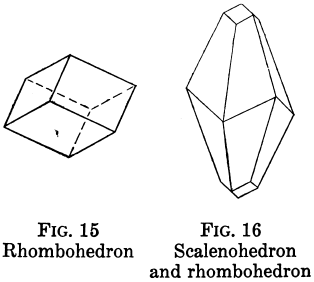
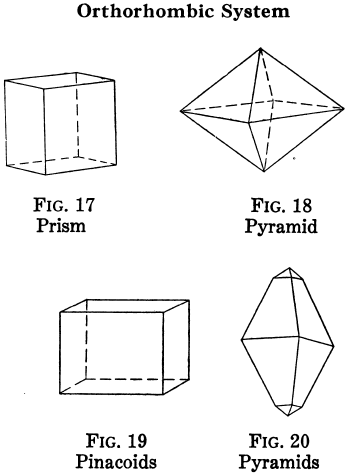
Orthorhombic Crystal Systems
Like I and II, ruled by right angles, but the properties of the crystals differ in all three directions.
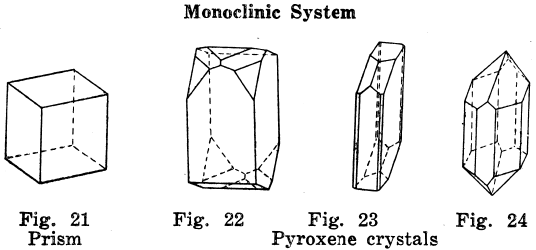
Monoclinic Crystal Systems
The plan of the crystals is such that it is off the square in one direction.
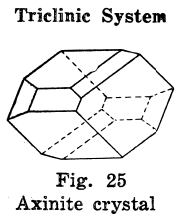
Triclinic Crystal Systems
The crystals in this system have departed altogether from the square plan.
Crystal Cleavage and Fracture
Cleavage is a property of crystals; it may be compared with the splitting of wood. Crystals of some minerals break in certain and definite directions, with smooth, flat surfaces, while others break irregularly in every direction; the former are said to have good cleavage, and the latter, no cleavage. Compare feldspar, which has good cleavage, with quartz, which has no cleavage. The irregular break or fracture, is described as uneven, hackly, or granular when it is rough, and as conchoidal when it is smooth and rounded, like a shell. The break may be flat and even, and very much like that of true cleavage; but close examination will reveal small hollows and elevations that are not seen on a true cleavage surface.
It should be noted that cleavage is a property of the single crystal, and that a mineral with good cleavage splits nicely only in certain and definite directions. If a specimen is made up of a great many small crystals having good cleavage, these crystals will be set at all sorts of angles relatively to the directions of good cleavage; so when the specimen is broken across, the cleavage will appear as smooth, flat patches, of a size depending on the size of the crystals, and set at various angles. On examining such specimens in a strong light, good cleavage surfaces will reflect like mirrors, as the specimen is turned very slowly. Try this with crystalline limestone or marble.
Crystal surfaces as originally formed are also usually flat, smooth, and good reflectors; they may be mistaken for cleavage surfaces.
Crystal Parting
Crystals of some minerals break with smooth, flat surfaces at some places; but this differs from true cleavage in the fact that between these places the break is rough and irregular. Corundum crystals have this property, which is called parting. Examine a specimen of corundum.
Crystal Twinning
Two or more crystals may grow together in certain definite ways, thus producing a crystal body that is called a twin (See Fig. 49). Grooves, or striations, and other irregularities on the surface, are sometimes due to twinning. (See Fig. 52.)
